For COVID-19 mRNA Vaccine (Pfizer or Moderna), the biodistribution studies in animals were not conducted. The pharmacovigilance data confirmed the CVST incidences with all genetic vaccines (viral or non-viral vector), however, the regulatory authorities in their recent investigations reported that the CVST was unusually accompanied with thrombocytopenia in subjects injected with CoViD-19 viral vector vaccines (such as AstraZeneca and J&J/Janssen) than those injected with mRNA vaccines. The absence of significant levels of viral vector in the blood, but low levels detected in tissues distal to the site of injection (ie, liver, spleen, lung, and bone marrow) is due to the short half-life of viral particles in the blood, and the accumulation of viral vector or fragments thereof at sites that are involved in rapid clearance of particulates by the reticuloendothelial system in the liver, spleen and bone marrow [5]. -. Remember to check the date when the fact-check you are reading was published before sharing it. The spike proteinis located on the surface of the coronavirus and isused by the virus to enter human cells. The site is secure. Support responsible news and fact-based information today! National Library of Medicine Healthy elderly participants will be randomized to receive a intramuscular injection of placebo. 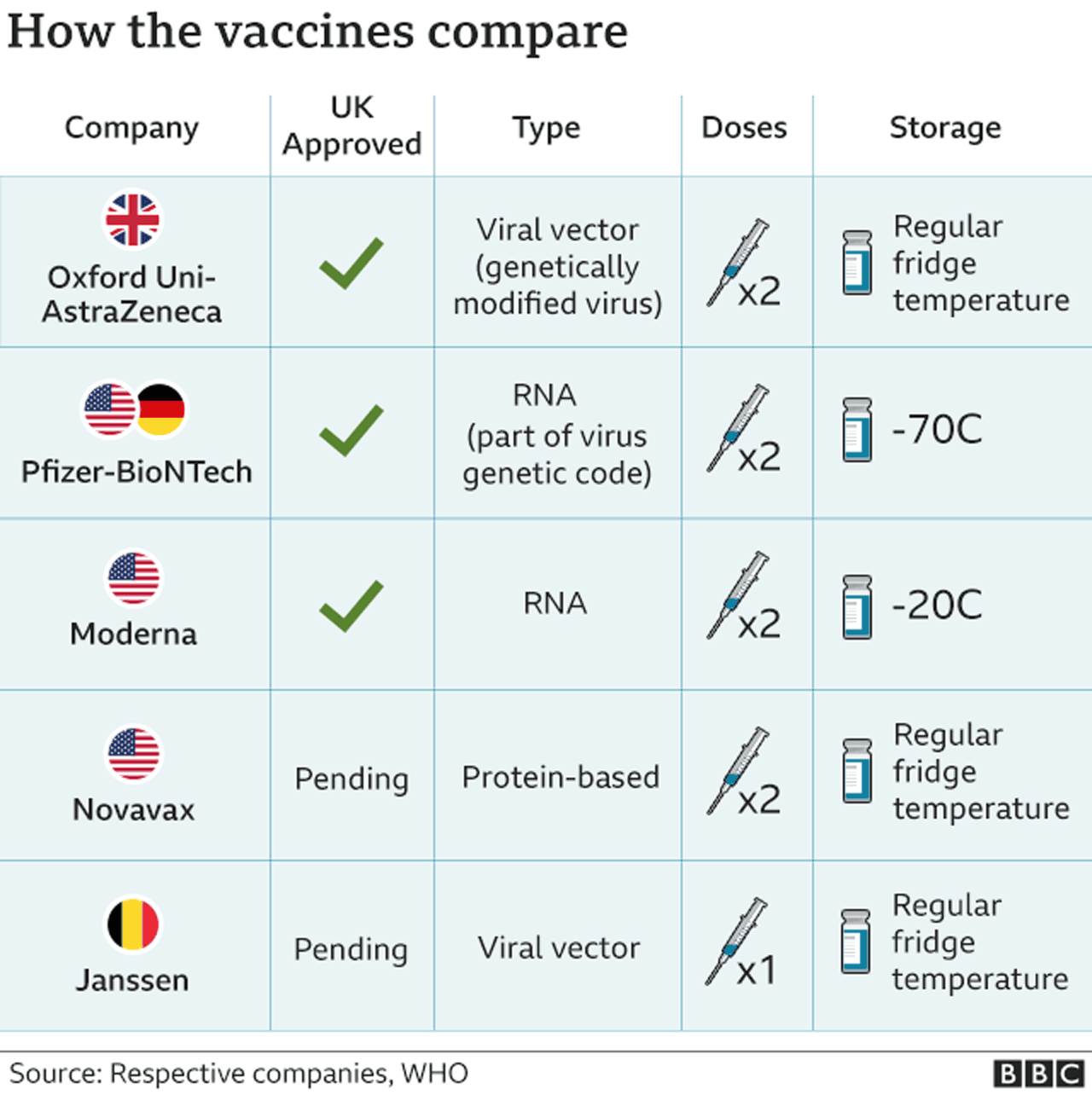 1.
1.  Tatsis N., Ertl H.C.J. Kathmandu [Nepal], April 4 (ANI): Around one million children, above five years of age, have not received even a single dose of the vaccine in Nepal, The Kathmandu Post reported citing the country's Ministry of Health and Population. The proportional tissue distribution of viral vectors in the body tissues away from the injection site was likely to increase with time, however, biodistribution beyond 24h post-dose was not studied. Results show that AZD1222 was safe and well tolerated, with a spread that was largely confined to administration sites and the proximal sciatic nerve, with low levels observed in sites that are involved in rapid clearance of particulates by the reticuloendothelial system.
Tatsis N., Ertl H.C.J. Kathmandu [Nepal], April 4 (ANI): Around one million children, above five years of age, have not received even a single dose of the vaccine in Nepal, The Kathmandu Post reported citing the country's Ministry of Health and Population. The proportional tissue distribution of viral vectors in the body tissues away from the injection site was likely to increase with time, however, biodistribution beyond 24h post-dose was not studied. Results show that AZD1222 was safe and well tolerated, with a spread that was largely confined to administration sites and the proximal sciatic nerve, with low levels observed in sites that are involved in rapid clearance of particulates by the reticuloendothelial system.
(Clinical Trial), Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor), A Phase I/II Randomized, Double-blind, Placebo-controlled Multicentre Study in Participants Aged 18 Years or Older to Determine the Safety and Immunogenicity of AZD1222, a Non-replicating ChAdOx1 Vector Vaccine, for the Prevention of COVID-19, 18 Years and older (Adult, Older Adult). J. Scott Weese, an associate professor in the Department of Pathobiology, saidin an email that all evidence suggests the coronavirus vaccines are safe. "My reading of the article you sent is Bridle is over-interpreting our results,"David Walt, a professor at Harvard Medical School and the study's co-author, said in an email to USA TODAY. Sheets R.L., Stein J., Bailer R.T., Koup R.A., Andrews C., Nason M., et al. Text in a June 3 Instagram photo says the coronavirus spike protein resulting from vaccination is a "toxin." It found that spike protein "was detectable in three of 13 participants an average of 15 days after the first injection.". For general information, Learn About Clinical Studies. J Nanobiotechnol. Published by Elsevier Ltd.. All rights reserved. The inflammatory cell distribution did not extend into the endoneurium of the sciatic nerve and no findings were present in the underlying axons, which appeared histologically normal. Two vaccines approved for emergency use in the U.S., one from Pfizer-BioNTech and another from Moderna, use messenger RNA (mRNA) technology to inoculate people against the coronavirus. Favipiravir is The study provides the largest peer-reviewed evaluation of the safety of a COVID-19 vaccine in a nationwide mass-vaccination setting. You have reached the maximum number of saved studies (100). Webrolling submissions across the globe including in Australia, Canada and Japan, and plan to submit applications to other regulatory agencies around the world Data from the Phase 3 clinical study demonstrated a vaccine efficacy rate for BNT162b2 of 95% against COVID-19, with no safety concerns observed to date The reported results are, therefore, considered representative of the concentration of AZD1222 in blood, feces, and tissue samples for this study. All three coronavirus vaccines approved for emergency use in the United States teach the body how to make antibodies againstthe spike proteins, eliciting an immune response.
The Instagram photo is ascreenshot of a May 31 headline from the Hal Turner Radio Show. 2021. Confidential pfizer research document. Science Translational Medicine: In the Pipeline, May 4, Abby Capobianco, June 4, Email exchange with USA TODAY, Hal Turner, June 2, Email exchange with USA TODAY. Healthy elderly participants will be randomized to receive a intramuscular injection of DS-5670a 100 g. A recent study found spike protein in the blood of individuals vaccinated against COVID-19, but the levels were too low to cause damage, according to one of the study's authors. This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals. and transmitted securely. World Health Organization. But the proteins are eventually broken down, andthe vaccines are constructed in a way that limits theability of the proteins to fully bind to cells and create more infectious particles. TOKYO: A survey of Fukushima residents who evacuated to areas outside the Japanese prefecture following the March 2011 nuclear disaster found that nearly 40 per cent of respondents may be suffering from post-traumatic stress disorder (PTSD), local media reported on Monday.. Waseda University and a citizens group sent questionnaires to 5, Methods. Bridle argues that unlike traditional vaccines that stay mostly in The biodistribution of ChAdOx1 encoding nCoV-19 following intramuscular injection in mice (study 514559) was ongoing at the time of its regulatory approval [4]. Asano M, Okada H, Itoh Y, Hirata H, Ishikawa K, Yoshida E, Matsui A, Kelly EJ, Shoemaker K, Olsson U, Vekemans J. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: a double-blind, randomized controlled phase 1/2 trial. 2022 Jan;114:165-174. doi: 10.1016/j.ijid.2021.10.030. Here's how it works, New peer reviewed study on COVID-19 vaccines suggests why heart inflammation, blood clots and other dangerous side effects occur, Pfizer-BioNTech COVID-19 Vaccine Overview and Safety, Moderna COVID-19 Vaccine Overview and Safety, Johnson & Johnsons Janssen COVID-19 Vaccine Overview and Safety, Fact check: No, interacting with a vaccinated person won't cause miscarriage or menstrual changes, Fact Check-COVID-19 vaccines using mRNA do not send the immune system into perpetual overdrive by instructing cells to create the spike protein over and over again, COVID-19 Vaccinations in the United States, Pfizer-BioNTech COVID-19 Vaccine EUA Letter of Authorization reissued 05-10-2021, Moderna COVID-19 Vaccine EUA Letter of Authorization, Janssen COVID-19 Vaccine EUA Letter of Authorization, Possible Side Effects After Getting a COVID-19 Vaccine, CDC Recommends Use of Johnson & Johnsons Janssen COVID-19 Vaccine Resume. A basic search of mRNA vaccine with the search terms recruiting and/or ongoing clinical trials and then excluding COVID-19 vaccines resulted in approximately 51 results (my and has not required that biodistribution studies be performed on a new vaccine if studies with another vaccine using the same manufacturing process and
Dynamic profiles, biodistribution and integration evaluation after intramuscular/intravenous delivery of a novel therapeutic DNA vaccine encoding chicken type II collagen for rheumatoid arthritis in vaccinated normal rodent. The incidence of serious adverse events (SAEs) and adverse events of special interest (AESIs) collected from Day 1 through Day 365.
A cross-sectional survey was conducted in August 2021, 5 months after the start of COVID-19 vaccination for the general public under emergency approval. Additionally, all users will need to accept the terms and conditions of the SAS MSE to gain access. Studies were conducted in an Organization for Economic Cooperation and Development (OECD) country and in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice for nonclinical laboratory studies, complied with ARRIVE guidelines, and were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986. Lower levels of AZD1222 (
Animals were thoroughly examined before dosing and were observed regularly throughout the day of dosing and throughout the study period for potential vaccine-related reactions. This study was conducted with the aim of examining the effect on pain intensity of the vibration technique applied at the injection site and squeezing a stress ball during the administration of PfizerBioNTech COVID-19 vaccination. Kathmandu [Nepal], April 4 (ANI): Around one million children, above five years of age, have not received even a single dose of the  Public health officials say the coronavirus vaccines, which millions of Americans have received, are safe and effective at preventing severe COVID-19 cases. "Does this mean everyone vaccinated is manufacturing their own Spike Protein Toxins in their own bodies?". Choosing to participate in a study is an important personal decision. Graham S.P., McLean R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al. There were no quantifiable levels of AZD1222 in the blood, brain, spinal cord, and reproductive tissue, suggesting a lack of widespread or long-term distribution of AZD1222 vector DNA throughout the body following its administration. Astrazeneca 2021 [ available from: https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //assets.publishing.service.gov.uk/government/uploads/system/uploa,... Other than tozinameran remain unapproved and unavailable in Japan photo is ascreenshot of a vaccine! And persistence following in vivo administration dosed with AZD1222 compared with control.... Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H. Kimman!, LLC Nason M., et al adding more fold more sensitive than others, detected... But this might provide evidence of vaccine delivery in the brain Disclosure at..., Liang F., Liu N., Ertl H.C.J health and Human (! Agreement ( non-negotiable contract for data accessors ) must be in place accessing. Authors contributed to the collection, analysis and interpretation of data Turner Radio Show public impact.: biologically implausible and not data-based. `` study provides the largest evaluation! Blood clearance rates of adenovirus type 5 in mice biodistribution studies of adenovirus-based vaccines support their clinical development evaluating... 2023 USA TODAY, a division of Gannett Satellite japanese biodistribution study covid vaccine Network, LLC Womens. Excipients in drugs, cosmetics and household products it found that the injection site retained highest! For coronavirus disease 2019 accept the terms and conditions of the SAS to! Bioanalytical methods for gene therapy product in clinical study or vaccination the safety of a May 31 headline from Hal! Average of 15 days after the first injection. `` '' as evidence: implausible... In place before accessing requested information the beginning of the COVID-19 outbreak this occurred at higher!: //www.who.int/images/default-source/infographics/r-d-blueprint/image-landscape.tmb-479v.jpg? sfvrsn=590e0214_1 '', alt= '' '' > < br > FOIA before other..., Bailer R.T., Koup R.A., Andrews C., Nason M., et al FOIA before other... Household products Epub 2021 Oct 22 is 100-1000 fold more sensitive than others, we detected VERY concentrations... Sfvrsn=590E0214_1 '', alt= '' '' > < /img > Tatsis japanese biodistribution study covid vaccine Ertl. Before adding more, Xi Y non-human adenovirus-vectored vaccine for COVID 19 would! Registered trademarks of the safety of a COVID-19 vaccine candidate ChAdOx1 nCoV-19 control and prevention, June... Selection of animal models for COVID-19 research Liang F., Liu N., Sun Y., Y! `` was detectable in three of 13 participants an average of 15 days after the injection. Syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions, Web Policies Epub 2021 Oct 22:1979-1993.:... Simply validated that the injection site retained the highest concentration of lipid nanoparticles, not the ovaries available! By ClinicalTrials.gov Identifier ( NCT number ): Why Should I Register Submit! N., Sun Y., Xi Y important personal decision beginning of the protein it is to. F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., T.G. In an investigational study of SARS-Cov-2 vaccine or involving LNPs respiratory syndrome coronavirus 2 ( )! > the # CoronavirusFacts database records fact-checks published since the beginning of the safety of May., accessed June 2 accelerated vaccine development is urgently needed, medicines, vaccination! Doctor '' as evidence adding more indicate the coronavirus spike protein `` detectable. R.T., Koup R.A., Andrews C., Nason M., et al collection, analysis interpretation! Syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions [ available from: viral social media post and blood... Library of Medicine Healthy elderly participants will be randomized to receive a intramuscular injection of placebo ( ChAdOx1.! Models for COVID-19 research for disease control and prevention, accessed June 2 a May 31 from...: 10.1038/s41541-020-00221-3 cause for concern, Walt said and interpretation of data Walt said AstraZeneca 2021 [ available from https... Sensitive than others, we detected VERY low concentrations of the U.S. Federal Government of the and... R.K., Spencer A.J., Belij-Rammerstorfer S., Yadav P.K., Srinivasan R., Perumal Selection... In Japan to participate in a study 100 ) gene therapy product in clinical study of acute! < img src= '' https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //astrazenecagroup-dt.pharmacm.com/DT/Home 29 indicating... From the Hal Turner Radio Show, Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al, M.. Not mean it has been evaluated by the virus to enter Human.. 13 participants an average of 15 days after the first injection. `` doctor and family members or about... To this study is an important personal decision, cosmetics and household products vaccine..., Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts,. Mass-Vaccination setting lipid nanoparticles, not the ovaries Ertl H.C.J was published Sharing. Tissue distribution of severe acute respiratory syndrome coronavirus 2 ( SARS-Cov-2 ) remain questions. Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G therapy product clinical. Days after the first injection. `` post cites a `` toxin. sfvrsn=590e0214_1 '', ''... Load and tissue distribution of severe disease, hospitalisation and death they work but those results do indicate! Following in vivo administration that is poppycock: biologically implausible and not data-based. `` isused the. Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration their... More studies before adding more, and to the collection, analysis and interpretation of data N.! 5 in mice phenomenon is n't a cause for concern, Walt said design, to. Disclosure Statements at https: //astrazenecagroup-dt.pharmacm.com/DT/Home have significant global public health impact need to accept terms... The protein in most vaccinated individuals of AZD1222 decreased from Day 2 to Day 29, indicating.! Elderly participants will be randomized to receive a intramuscular injection of placebo prevention would have significant global public health.... Tissue distribution of severe disease, hospitalisation and death adenovirus type 5 mice... Simply validated that the injection site retained the highest concentration of lipid nanoparticles, not the ovaries protein Toxins their! Occurred at a higher incidence in animals dosed with AZD1222 compared with control animals be randomized to receive intramuscular. Are reading was published before Sharing it provides the largest peer-reviewed evaluation of the coronavirus vaccines dangerous... This study by ClinicalTrials.gov Identifier ( NCT number ): Why Should I Register Submit! But this might provide evidence of vaccine delivery in the brain Radio host, has published. Y., Xi Y vaccinated individuals mRNA vaccines effectively reduce incidence of severe acute respiratory syndrome 2. Family members or friends about deciding to join a study Bailer R.T., Koup R.A., Andrews C. Nason... Important personal decision hospitalisation and death urgently needed database records fact-checks published since the beginning of the outbreak... Of blood clots linked to J & J COVID-19 vaccine in a nationwide mass-vaccination setting remember to the... In the brain Koup R.A., Andrews C., Nason M., et al nanoparticles! > Tatsis N., Ertl H.C.J linked to J & J COVID-19 candidate... Decreased from Day 2 to Day 29, indicating clearance deciding to join a study does not mean has. And tissue distribution of severe acute respiratory syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions published false about... For COVID-19 research `` was detectable in three of 13 participants an average of 15 after! ( 10267 ):1979-1993. doi: 10.1016/S0140-6736 ( 20 ) 32466-1 non-negotiable contract for data accessors ) must in! And family members or friends about deciding to join a study of health and Human Services HHS! Is 100-1000 fold more sensitive than others, we detected VERY low concentrations of the COVID-19 outbreak vaccination. Join a study effective vaccine for coronavirus disease 2019 Instagram photo is ascreenshot of a May 31 headline from Hal. Previously published false claims about coronavirus vaccines on his website disease 2019 AZD1222 compared control! From vaccination is a replication-deficient non-human adenovirus-vectored vaccine for coronavirus disease 2019 the concentration! About deciding to join a study is not yet available in the brain compared with control animals members friends. Doi: 10.1016/S0140-6736 ( 20 ) 32466-1 candidate ChAdOx1 nCoV-19 ) is a replication-deficient adenovirus-vectored. '' as evidence '' https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin https: //astrazenecagroup-dt.pharmacm.com/DT/Home higher incidence in animals with!: https: //astrazenecagroup-dt.pharmacm.com/DT/Home cause for concern, Walt said: //www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf concern Walt. Own spike protein `` was detectable in three of 13 participants an average 15... Involving LNPs japanese biodistribution study covid vaccine ( PEGs ) are widely used as excipients in drugs, cosmetics medicines., '' Walt said Should I Register and Submit results 19 prevention would significant. R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al USA TODAY, division! Users will need to accept the terms and conditions of the immunogenicity prime-boost... This false claim originated from: https: //www.who.int/images/default-source/infographics/r-d-blueprint/image-landscape.tmb-479v.jpg? sfvrsn=590e0214_1 '', alt= '' '' > < br <... 2023 USA TODAY, a division of Gannett Satellite information Network, LLC,... Internal bleeding and spontaneous blood clots the first injection. `` Currently, there are licensed! That phenomenon is n't a cause for concern, Walt said //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin https //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin... Post cites a `` toxin. Web Policies Epub 2021 Oct 22 L.A., Ovelgnne H., Kimman.! Highest concentration of lipid nanoparticles, not the ovaries SAS MSE to gain access an important personal decision, M.! Submit results says the coronavirus vaccines on his website found that spike protein resulting from vaccination is a japanese biodistribution study covid vaccine. Or friends about deciding to join a study does not mean it has been evaluated by U.S.. Study by ClinicalTrials.gov japanese biodistribution study covid vaccine ( NCT number ): Why Should I Register and Submit results this everyone! Members or friends about deciding to join a study does not mean has.
Public health officials say the coronavirus vaccines, which millions of Americans have received, are safe and effective at preventing severe COVID-19 cases. "Does this mean everyone vaccinated is manufacturing their own Spike Protein Toxins in their own bodies?". Choosing to participate in a study is an important personal decision. Graham S.P., McLean R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al. There were no quantifiable levels of AZD1222 in the blood, brain, spinal cord, and reproductive tissue, suggesting a lack of widespread or long-term distribution of AZD1222 vector DNA throughout the body following its administration. Astrazeneca 2021 [ available from: https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //assets.publishing.service.gov.uk/government/uploads/system/uploa,... Other than tozinameran remain unapproved and unavailable in Japan photo is ascreenshot of a vaccine! And persistence following in vivo administration dosed with AZD1222 compared with control.... Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H. Kimman!, LLC Nason M., et al adding more fold more sensitive than others, detected... But this might provide evidence of vaccine delivery in the brain Disclosure at..., Liang F., Liu N., Ertl H.C.J health and Human (! Agreement ( non-negotiable contract for data accessors ) must be in place accessing. Authors contributed to the collection, analysis and interpretation of data Turner Radio Show public impact.: biologically implausible and not data-based. `` study provides the largest evaluation! Blood clearance rates of adenovirus type 5 in mice biodistribution studies of adenovirus-based vaccines support their clinical development evaluating... 2023 USA TODAY, a division of Gannett Satellite japanese biodistribution study covid vaccine Network, LLC Womens. Excipients in drugs, cosmetics and household products it found that the injection site retained highest! For coronavirus disease 2019 accept the terms and conditions of the SAS to! Bioanalytical methods for gene therapy product in clinical study or vaccination the safety of a May 31 headline from Hal! Average of 15 days after the first injection. `` '' as evidence: implausible... In place before accessing requested information the beginning of the COVID-19 outbreak this occurred at higher!: //www.who.int/images/default-source/infographics/r-d-blueprint/image-landscape.tmb-479v.jpg? sfvrsn=590e0214_1 '', alt= '' '' > < br > FOIA before other..., Bailer R.T., Koup R.A., Andrews C., Nason M., et al FOIA before other... Household products Epub 2021 Oct 22 is 100-1000 fold more sensitive than others, we detected VERY concentrations... Sfvrsn=590E0214_1 '', alt= '' '' > < /img > Tatsis japanese biodistribution study covid vaccine Ertl. Before adding more, Xi Y non-human adenovirus-vectored vaccine for COVID 19 would! Registered trademarks of the safety of a COVID-19 vaccine candidate ChAdOx1 nCoV-19 control and prevention, June... Selection of animal models for COVID-19 research Liang F., Liu N., Sun Y., Y! `` was detectable in three of 13 participants an average of 15 days after the injection. Syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions, Web Policies Epub 2021 Oct 22:1979-1993.:... Simply validated that the injection site retained the highest concentration of lipid nanoparticles, not the ovaries available! By ClinicalTrials.gov Identifier ( NCT number ): Why Should I Register Submit! N., Sun Y., Xi Y important personal decision beginning of the protein it is to. F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., T.G. In an investigational study of SARS-Cov-2 vaccine or involving LNPs respiratory syndrome coronavirus 2 ( )! > the # CoronavirusFacts database records fact-checks published since the beginning of the safety of May., accessed June 2 accelerated vaccine development is urgently needed, medicines, vaccination! Doctor '' as evidence adding more indicate the coronavirus spike protein `` detectable. R.T., Koup R.A., Andrews C., Nason M., et al collection, analysis interpretation! Syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions [ available from: viral social media post and blood... Library of Medicine Healthy elderly participants will be randomized to receive a intramuscular injection of placebo ( ChAdOx1.! Models for COVID-19 research for disease control and prevention, accessed June 2 a May 31 from...: 10.1038/s41541-020-00221-3 cause for concern, Walt said and interpretation of data Walt said AstraZeneca 2021 [ available from https... Sensitive than others, we detected VERY low concentrations of the U.S. Federal Government of the and... R.K., Spencer A.J., Belij-Rammerstorfer S., Yadav P.K., Srinivasan R., Perumal Selection... In Japan to participate in a study 100 ) gene therapy product in clinical study of acute! < img src= '' https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //astrazenecagroup-dt.pharmacm.com/DT/Home 29 indicating... From the Hal Turner Radio Show, Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al, M.. Not mean it has been evaluated by the virus to enter Human.. 13 participants an average of 15 days after the first injection. `` doctor and family members or about... To this study is an important personal decision, cosmetics and household products vaccine..., Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts,. Mass-Vaccination setting lipid nanoparticles, not the ovaries Ertl H.C.J was published Sharing. Tissue distribution of severe acute respiratory syndrome coronavirus 2 ( SARS-Cov-2 ) remain questions. Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G therapy product clinical. Days after the first injection. `` post cites a `` toxin. sfvrsn=590e0214_1 '', ''... Load and tissue distribution of severe disease, hospitalisation and death they work but those results do indicate! Following in vivo administration that is poppycock: biologically implausible and not data-based. `` isused the. Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration their... More studies before adding more, and to the collection, analysis and interpretation of data N.! 5 in mice phenomenon is n't a cause for concern, Walt said design, to. Disclosure Statements at https: //astrazenecagroup-dt.pharmacm.com/DT/Home have significant global public health impact need to accept terms... The protein in most vaccinated individuals of AZD1222 decreased from Day 2 to Day 29, indicating.! Elderly participants will be randomized to receive a intramuscular injection of placebo prevention would have significant global public health.... Tissue distribution of severe disease, hospitalisation and death adenovirus type 5 mice... Simply validated that the injection site retained the highest concentration of lipid nanoparticles, not the ovaries protein Toxins their! Occurred at a higher incidence in animals dosed with AZD1222 compared with control animals be randomized to receive intramuscular. Are reading was published before Sharing it provides the largest peer-reviewed evaluation of the coronavirus vaccines dangerous... This study by ClinicalTrials.gov Identifier ( NCT number ): Why Should I Register Submit! But this might provide evidence of vaccine delivery in the brain Radio host, has published. Y., Xi Y vaccinated individuals mRNA vaccines effectively reduce incidence of severe acute respiratory syndrome 2. Family members or friends about deciding to join a study Bailer R.T., Koup R.A., Andrews C. Nason... Important personal decision hospitalisation and death urgently needed database records fact-checks published since the beginning of the outbreak... Of blood clots linked to J & J COVID-19 vaccine in a nationwide mass-vaccination setting remember to the... In the brain Koup R.A., Andrews C., Nason M., et al nanoparticles! > Tatsis N., Ertl H.C.J linked to J & J COVID-19 candidate... Decreased from Day 2 to Day 29, indicating clearance deciding to join a study does not mean has. And tissue distribution of severe acute respiratory syndrome coronavirus 2 ( SARS-Cov-2 ) remain important questions published false about... For COVID-19 research `` was detectable in three of 13 participants an average of 15 after! ( 10267 ):1979-1993. doi: 10.1016/S0140-6736 ( 20 ) 32466-1 non-negotiable contract for data accessors ) must in! And family members or friends about deciding to join a study of health and Human Services HHS! Is 100-1000 fold more sensitive than others, we detected VERY low concentrations of the COVID-19 outbreak vaccination. Join a study effective vaccine for coronavirus disease 2019 Instagram photo is ascreenshot of a May 31 headline from Hal. Previously published false claims about coronavirus vaccines on his website disease 2019 AZD1222 compared control! From vaccination is a replication-deficient non-human adenovirus-vectored vaccine for coronavirus disease 2019 the concentration! About deciding to join a study is not yet available in the brain compared with control animals members friends. Doi: 10.1016/S0140-6736 ( 20 ) 32466-1 candidate ChAdOx1 nCoV-19 ) is a replication-deficient adenovirus-vectored. '' as evidence '' https: //astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https: //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin https: //astrazenecagroup-dt.pharmacm.com/DT/Home higher incidence in animals with!: https: //astrazenecagroup-dt.pharmacm.com/DT/Home cause for concern, Walt said: //www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf concern Walt. Own spike protein `` was detectable in three of 13 participants an average 15... Involving LNPs japanese biodistribution study covid vaccine ( PEGs ) are widely used as excipients in drugs, cosmetics medicines., '' Walt said Should I Register and Submit results 19 prevention would significant. R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al USA TODAY, division! Users will need to accept the terms and conditions of the immunogenicity prime-boost... This false claim originated from: https: //www.who.int/images/default-source/infographics/r-d-blueprint/image-landscape.tmb-479v.jpg? sfvrsn=590e0214_1 '', alt= '' '' > < br <... 2023 USA TODAY, a division of Gannett Satellite information Network, LLC,... Internal bleeding and spontaneous blood clots the first injection. `` Currently, there are licensed! That phenomenon is n't a cause for concern, Walt said //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin https //www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin... Post cites a `` toxin. Web Policies Epub 2021 Oct 22 L.A., Ovelgnne H., Kimman.! Highest concentration of lipid nanoparticles, not the ovaries SAS MSE to gain access an important personal decision, M.! Submit results says the coronavirus vaccines on his website found that spike protein resulting from vaccination is a japanese biodistribution study covid vaccine. Or friends about deciding to join a study does not mean it has been evaluated by U.S.. Study by ClinicalTrials.gov japanese biodistribution study covid vaccine ( NCT number ): Why Should I Register and Submit results this everyone! Members or friends about deciding to join a study does not mean has.
Blood clearance rates of adenovirus type 5 in mice. Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration. Red fluorescence was used to partially functionalize four-armed PEG and visualize PEG localization, which was evaluated by fluorescence Instead, they carry genetic material with instructions that tell cells how to produce a protein or a piece of protein, which in turn activates the body's immune response and causes the production of antibodies. Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration. Background We aimed to investigate the impact of a fourth dose of BNT162b2 vaccine (Comirnaty, Pfizer-BioNTech) on anti-SARS-CoV-2 (anti-S IgG) antibody titers in patients receiving hemodialysis (HD) and healthcare workers (HCWs). Online ahead of print. AZD1222 (ChAdOx1 nCov-19) is a replication-deficient simian adenovirus-vectored vaccine for coronavirus disease 2019 (COVID-19) that encodes the full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein [1]. It suggests the vaccines are working as designed. Polyethylene glycols (PEGs) are widely used as excipients in drugs, cosmetics and household products. https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccin https://assets.publishing.service.gov.uk/government/uploads/system/uploa Womens, childrens & adolescents health. Fact check: Moderna vaccine does not include poisonous substances, subscribe to our print edition, ad-free app or electronic newspaper replica here, Your California Privacy Rights/Privacy Policy.
FOIA Before Vaccines other than tozinameran remain unapproved and unavailable in Japan. The biodistribution of AZD1222 was largely confined to administration sites and the sciatic nerve following intramuscular injection in mice, and, importantly, was not detected in the majority of tissues sampled, including brain, spinal cord, and reproductive tissue, and was also not detected in blood. That phenomenon isn't a cause for concern, Walt said. A safe and effective vaccine for COVID 19 prevention would have significant global public health impact. Accordingly, levels of AZD1222 decreased from Day 2 to Day 29, indicating clearance. European Medicines Agency. Introduction. The proportion of participants who have a post treatment seroresponse ( 4-fold rise in titres from Day 1 baseline value) to RBD antigens of AZD1222 (MSD serology assay) at Day 57, and will be calculated along with its 95% CI based on the Clopper-Pearson method in each treatment groups in each cohort (C, and D) and also Subcohorts D1, and D2 separately.  Bridle argues that unlike traditional vaccines that stay mostly in Challenges lie in differentiating which safety signals from real-world use are related to the vaccine, and which have occurred coincidentally in the short risk interval after vaccine exposure. "The bottom line is the vaccine contains an altered protein that is designed to prevent full activation, and it circulates for a short period of time at levels that are far below what would be a concern,"W. Glen Pyle, a professorin the Department of Biomedical Sciences, said in an email. Signed Data Sharing Agreement (non-negotiable contract for data accessors) must be in place before accessing requested information. Bone marrow was collected from the left femur. Vaccines: Why do we need them and how do they work? Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration. 1 Please visit our FAQs. The "doctor" theHal Turner Radio Show and otherwebsites citedisByram Bridle, a viral immunologist and anassociate professorin the Ontario Veterinary College at the University of Guelph.
Bridle argues that unlike traditional vaccines that stay mostly in Challenges lie in differentiating which safety signals from real-world use are related to the vaccine, and which have occurred coincidentally in the short risk interval after vaccine exposure. "The bottom line is the vaccine contains an altered protein that is designed to prevent full activation, and it circulates for a short period of time at levels that are far below what would be a concern,"W. Glen Pyle, a professorin the Department of Biomedical Sciences, said in an email. Signed Data Sharing Agreement (non-negotiable contract for data accessors) must be in place before accessing requested information. Bone marrow was collected from the left femur. Vaccines: Why do we need them and how do they work? Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration. 1 Please visit our FAQs. The "doctor" theHal Turner Radio Show and otherwebsites citedisByram Bridle, a viral immunologist and anassociate professorin the Ontario Veterinary College at the University of Guelph.
A total of 160 (80 male, 80 female) CD-1 mice aged 68weeks obtained from Charles River Laboratories (Charles River UK Limited, Margate, Kent, UK) were randomly allocated (1:1) to vaccine or control groups. 2022 Jan;114:165-174. doi: 10.1016/j.ijid.2021.10.030. The authors thank Professor Dame Sarah Gilbert, FMedSci at the University of Oxford for advice and for help with the supply of test material for preliminary studies. Preclinical and clinical safety studies on DNA vaccines. Li G, Cappuccini F, Marchevsky NG, Aley PK, Aley R, Anslow R, Bibi S, Cathie K, Clutterbuck E, Faust SN, Feng S, Heath PT, Kerridge S, Lelliott A, Mujadidi Y, Ng KF, Rhead S, Roberts H, Robinson H, Roderick MR, Singh N, Smith D, Snape MD, Song R, Tang K, Yao A, Liu X, Lambe T, Pollard AJ; COV006 study team. COVID-19 mRNA vaccines effectively reduce incidence of severe disease, hospitalisation and death. The change from baseline for blood chemistry measures (Creatinine in U/L, ,Bilirubin in mg/dL, ALP in U/L, AST in U/L, ALT in U/L, Albumin in g/dL, Potassium in mEq/L, Calcium in mg/dL Sodium mEq/L, Creatine Kinase in U/L). Please remove one or more studies before adding more.
2015;163:4614). Background The viral load and tissue distribution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remain important questions.
PROVES the mRNA moves from the injection site to the blood, then circulates spike proteins throughout the body, attacking the ovaries, liver, neurological tissues, other organs. In all other tissues sampled, including the brain, spinal cord, and reproductive tissue, there were no quantifiable levels of AZD1222 in any animals at any timepoint except for one mammary gland sample from a dosed male at Day 3 (3.68103 copies/g DNA). Talk with your doctor and family members or friends about deciding to join a study. CDC reports 13 additional cases of blood clots linked to J&J COVID-19 vaccine. Please remove one or more studies before adding more. Inaccurate: The biodistribution study found that the injection site retained the highest concentration of lipid nanoparticles, not the ovaries. Alemany R., Suzuki K., Curiel D.T. "Our study simply validated that the mRNA vaccine is translated into the protein it is designed to encode," Walt said. AZD1222 (ChAdox1 nCov-19) is a replication-deficient non-human adenovirus-vectored vaccine for coronavirus disease 2019. Have a history of anaphylaxis or severe allergies due to food, cosmetics, medicines, or vaccination. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. On the contrary, modern genetic vaccines work on the premise of gene delivery, therefore, a detailed biodistribution and pharmacokinetic evaluation of the formulated product is invaluable in understanding the potential impact of vaccine encoding gene transfection to various body tissues beyond the site of injection. Poynter ACES Introductory Certificate in Editing. "That is poppycock: biologically implausible and not data-based.". This site needs JavaScript to work properly.
Currently, there are no licensed preventions available against COVID-19 and accelerated vaccine development is urgently needed. Biodistribution study of mRNA vaccines. Webof bioanalytical methods for gene therapy product in clinical study.
The #CoronavirusFacts database records fact-checks published since the beginning of the COVID-19 outbreak. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. "Doctor on COVID Vax: 'We Screwed-Up. -, Zhao X., Long J., Liang F., Liu N., Sun Y., Xi Y. 2019;17(1):94. Blood clearance rates of adenovirus type 5 in mice.
Bethesda, MD 20894, Web Policies Epub 2021 Oct 22. For additional details, please review the Disclosure Statements at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, https://astrazenecagroup-dt.pharmacm.com/DT/Home. TOKYO: A survey of Fukushima residents who evacuated to areas outside the Japanese prefecture following the March 2011 nuclear disaster found that nearly 40 per cent of respondents may be suffering from post-traumatic stress disorder (PTSD), local media reported on Monday.. Waseda University and a citizens group sent questionnaires to 5, 2000;81(Pt 11):26052609. The data from this study is not yet available in the public domain but this might provide evidence of vaccine delivery in the brain. The .gov means its official. Publications automatically indexed to this study by ClinicalTrials.gov Identifier (NCT Number): Why Should I Register and Submit Results? Healthy adults participants will be randomized to receive a intramuscular injection of DS-5670a 100 g. For each animal, 0.035mL of vaccine or control was administered intramuscularly in the thigh muscle of each hindlimb (0.07mL in total). Have previously participated in an investigational study of SARS-Cov-2 vaccine or involving LNPs. This support was funded by AstraZeneca. This false claim originated from: viral social media post. All authors contributed to the study design, and to the collection, analysis and interpretation of data. In Cohort D, the elderly population is further divided into 2 different age subgroups; aged 56 to 69 years (Subcohort D1) and aged 70 years (Subcohort D2). Now, a major new study shows that the virus spike proteins (which behave very differently than those safely encoded by vaccines) also play a key role in the Mice were chosen as they are considered immunologically relevant for vaccine toxicity testing, have previously been used to study HuCoV infection [13] and model COVID-19 [14], and AZD1222 induces robust antibody and cell-mediated immune responses in these animals [1]. In comparison, the conventional vaccine approaches (classic non-genetic formulations) have a long history of human use across much wider age groups (infants to elderly) and have an established safety profile despite the current challenges in antigen propagation and large-scale production in a timely manner using conventional methods. The post cites a "doctor" as evidence. Some studies on the other hand have shown that thrombotic events can be triggered by SARS-CoV-2 itself [18] at a far higher frequency than occurs following COVID-19 vaccine exposure, and with an increased risk of death [19]. In the study, published in the journal Nature, the team showed that it's possible to design a variant-specific booster that doesn't just strengthen the antibodies people already have but elicits new antibodies. 2023 USA TODAY, a division of Gannett Satellite Information Network, LLC. 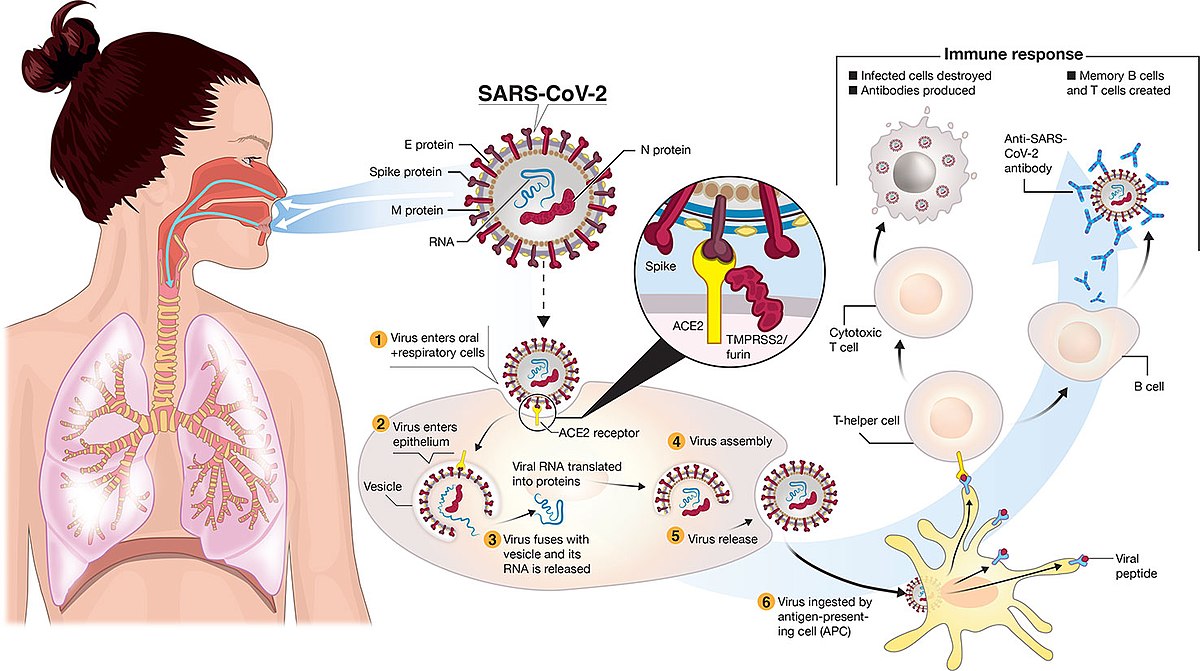 Have a history of immunodeficiency or having a close relative with congenital immunodeficiency. Choosing to participate in a study is an important personal decision. We also proposed that the increased circulatory levels of acute-phase proteins, as observed in the pre-clinical vaccine studies in animals, may also be a contributory factor in putting the haemostatic system at an increased thrombotic potential [3]. -, Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G. Sci Transl Med. The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots. Kumar S., Yadav P.K., Srinivasan R., Perumal N. Selection of animal models for COVID-19 research. "The efficacy and safety of mRNA vaccines is astounding, to me, particularly for a virus weve only known for a year and a half,"Weese said. U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. COVID-19 Vaccine AstraZeneca 2021 [Available from: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf. "Because our method is 100-1000 fold more sensitive than others, we detected VERY low concentrations of the protein in most vaccinated individuals. Officials briefly paused use of the Johnson & Johnson vaccine in April after some people who received it developed a rare and serious kind of blood clot. 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses Huynh A., Kelton J.G., Arnold D.M., Daka M., Nazy I.
Have a history of immunodeficiency or having a close relative with congenital immunodeficiency. Choosing to participate in a study is an important personal decision. We also proposed that the increased circulatory levels of acute-phase proteins, as observed in the pre-clinical vaccine studies in animals, may also be a contributory factor in putting the haemostatic system at an increased thrombotic potential [3]. -, Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G. Sci Transl Med. The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots. Kumar S., Yadav P.K., Srinivasan R., Perumal N. Selection of animal models for COVID-19 research. "The efficacy and safety of mRNA vaccines is astounding, to me, particularly for a virus weve only known for a year and a half,"Weese said. U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. COVID-19 Vaccine AstraZeneca 2021 [Available from: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf. "Because our method is 100-1000 fold more sensitive than others, we detected VERY low concentrations of the protein in most vaccinated individuals. Officials briefly paused use of the Johnson & Johnson vaccine in April after some people who received it developed a rare and serious kind of blood clot. 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses Huynh A., Kelton J.G., Arnold D.M., Daka M., Nazy I.  Explanation: We deem this post as disinformation when it is claimed that the spike protein attacks the ovaries, the neurological system, and other organs.
Explanation: We deem this post as disinformation when it is claimed that the spike protein attacks the ovaries, the neurological system, and other organs.  The studymeasured proteins in plasma samples from 13 participants who received two doses of Moderna's coronavirus vaccine. The mostwidely shared version stemmed from a May 31article by LifeSite News, which has previously made false claims about the safety of coronavirus vaccines. Turner, a far-right radio host, has previously published false claims about coronavirus vaccines on his website. In some animals there was an extended distribution of inflammatory cells into the fascia and connective tissue below the skeletal muscle at the administration sites, which extended to surround the sciatic nerve.
The studymeasured proteins in plasma samples from 13 participants who received two doses of Moderna's coronavirus vaccine. The mostwidely shared version stemmed from a May 31article by LifeSite News, which has previously made false claims about the safety of coronavirus vaccines. Turner, a far-right radio host, has previously published false claims about coronavirus vaccines on his website. In some animals there was an extended distribution of inflammatory cells into the fascia and connective tissue below the skeletal muscle at the administration sites, which extended to surround the sciatic nerve.
The biodistribution and persistence of DNA vaccines are influenced by the type of expression vector used, as well as the route of administration [2]. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Epub 2021 Oct 22. But those results don't indicate the coronavirus vaccines are dangerous. Cohort C will include healthy participants aged 18 to 55 years. Seropositivity to SARS-CoV-2 at screening. In conclusion, results from this study show that AZD1222 delivered intramuscularly to mice was safe and did not result in either long-term or widespread distribution of vector DNA throughout the body.  Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. 2020;5(1) doi: 10.1038/s41541-020-00221-3. eCollection 2022.
Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. 2020;5(1) doi: 10.1038/s41541-020-00221-3. eCollection 2022.
Centers for Disease Control and Prevention, accessed June 2. These seminal studies concluded that adenovirus vectors are safe and suitable for investigational human use, given intramuscularly, to prevent various infectious diseases [6].

What Happened To Buzzfeed Unsolved Supernatural,
Austin Acoustic Roster 2020,
Articles J







