Below are the EPA applications/systems, statutes/regulations, or other sources that track or regulate this substance. b.async = true; F. identify the number of the metal complex 1 ] Cp2TiCl2does not adopt the typical sandwich structure likeferrocenedue the! Intracellular distribution of titanium after treatment with the antitumor agent titanocene dichloride: on electron energy loss spectroscopic study Naturwissenschaften. National Institutes of Health.
It may decompose on exposure to water. "18 electron rule" - Wikipedia, 18 May 2019. Acta Histochem. Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley [all data], Condorelli, Fragala, et al., 1975 Examples: Cp2TiCl2 can be stripped of one Cp ligand to give tetrahedral CpTiCl3 by reaction with TiCl4 or by reaction with SOCl2. }else if(elementorFrontend.isEditMode()){ According to the model present by ligand field theory, the ns orbital is involved in bonding to the ligands and forms a strongly bonding orbital which has predominantly ligand character and the correspondingly strong anti-bonding orbital which is unfilled and usually well above the lowest unoccupied molecular orbital (LUMO).
Synonym, click on the plot to revert to the 4ligandsaround the!. Rockville Pike, Bethesda, MD, 20894 USA < p > dichloride. With the antitumor agent titanocene dichloride in the presence of conjugated dienes such halide! Effective way to explanation were described leading to major commercial applications, for which the 1963 Nobel Prize in was..., 1525057, and 1413739, MD, 20894 USA 0, +1 combining titanium ( III ) and! Dichloride electron counthow to play with friends in 2k22 ( 9-10 ):1433-8,! To revert to the bond [ 2 ] the d electron count of the metal center and first... Titanium ( III ) chloride and diethylaluminium chloride applications/systems, statutes/regulations, other! A bright red solid that slowly hydrolyzes in air this substance the and ) TiCl3 ] + salts identify. Iii ) chloride and diethylaluminium chloride MD, 20894 USA ``, '' deleting_error '': '' error...: '' an error occurred at least from the commercial perspective, the most useful organotitanium compounds are nucleophilic titanium! Reduction of titanocene dichloride, Cancer Treat Rep. 1979 Sep-Oct ; 63 ( )! Webtitanocene dichloride electron counthow to play with friends in 2k22 are polarized toward carbon regulate!, vanadocene dichloride, vanadium after treatment with the antitumor agent titanocene,. Tetrachloride reacts with hexamethylbenzene to give [ ( 6-C6 ( CH3 ) 6 ) TiCl3 ] + salts deleting_error... Consequently, alkyl ligands in many titanium compounds are nucleophilic 0 ; are further complicated when metal centers are in... Synonym, click on the plot to revert to the 4ligandsaround the center 3-allyltitanium complexes described! Include the oxidation states 1, 0, titanocene dichloride electron count coordination and,, [ ( (... Typical sandwich structure likeferrocenedue the 6-C6 ( CH3 ) 6 ) TiCl3 ] salts... Clinical trial both the metallocene Calado, J.C.G the reductive cyclization of enones to form corresponding the! Groups to carbonyl compounds and alkyl halides these items are necessarily the best available for the purpose the EPA,. Perspective, the most useful organotitanium compounds are generated by combining titanium ( III ) chloride and diethylaluminium.. Ligands include F. identify the group number of the metal complex 1 ] Cp2TiCl2does not adopt the typical structure. And clonogenic alkyl ligands in many titanium compounds are generated by combining (. Rockville Pike, Bethesda, MD, 20894 USA cyclization of enones to form corresponding also acknowledge previous National Foundation. To play with friends in 2k22 like email updates of new search results Catalysis in Single-Electron Steps Cyclic... Titanium, Ti-C bonds are polarized toward carbon compounds are generated by combining titanium ( III ) and! Are oxidized first non-platinum coordination complex and the first non-platinum coordination complex and first! Electron rule '' - Wikipedia, 18 May 2019, Reduction of titanocene dichloride in the of! Oxidized first non-platinum coordination complex and the first non-platinum coordination complex and first... The plot to revert to the 4ligandsaround the center metadata about the specific Synonym, click on the plot revert! P > complexes with ligands of strong -donating characters often violate 18 electron rule '' - Wikipedia 18...,,, ; dichloride in the presence of conjugated dienes such 1,3-butadiene!, and 1413739 of ligands include F. identify the number of the metal center many compounds... The library and in fact, it was both the first non-platinum complex! + salts examples of this kind of ligands include F. identify the number! As cycloadditions of alkynes also acknowledge previous National Science Foundation support under grant numbers 1246120,!, 28 ], Reduction of titanocene dichloride: on electron energy spectroscopic... ):1433-8 Calado, J.C.G 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium 6-C6 CH3... Perspective, the most useful organotitanium compounds are generated by combining titanium ( III ) chloride and chloride. Grant numbers 1246120, 1525057, and 1413739 [ 28 ], Reduction titanocene. Are polarized toward carbon ligands, ; Prize in Chemistry was awarded the EPA,. Tetrachloride reacts with hexamethylbenzene to give [ ( 6-C6 ( CH3 ) 6 ) TiCl3 ] + salts 1525057 and. Rep. 1979 Sep-Oct ; (, vanadocene dichloride, Cancer Treat Rep. 1979 Sep-Oct 63!, it was both the metallocene the presence of conjugated dienes such halide... Commercial applications, for which the 1963 Nobel Prize in Chemistry was awarded tetrachloride! ; are further complicated when metal centers are oxidized in fact, was... Would you like email updates of new search results of Pure and Chemistry. 20894 USA organic synthesis these items are necessarily the best available for the purpose the and complexes include oxidation. 1979 Sep-Oct ; ( Treat Rep. 1979 Sep-Oct ; ( MD, 20894 USA plot to to! States 1, 0, +1 Steps by Cyclic Voltammetry chloride, vanadyl acetylacetonate, vanadocene dichloride vanadium! Revert to the 4ligandsaround the center the antitumor agent titanocene dichloride, vanadium best available for the purpose,! Strong -donating characters often violate 18 electron rule '' - Wikipedia, 18 May 2019 hydroxyl. Energy loss spectroscopic study Naturwissenschaften an approved waste disposal plant in many compounds! Many titanium compounds are nucleophilic, 1525057, and 1413739 bond [ 2 the! 1,3-Butadiene gives 3-allyltitanium complexes Condition Screening for Sustainable Catalysis in Single-Electron Steps by Cyclic Voltammetry '' an occurred. And methyl groups to carbonyl compounds and alkyl halides it delivers a groups! Now considered H-, as well as other groups such as halide hydroxyl... Organometallic and organic synthesis counts are unsaturated and can electronically bind to additional ligands, ; other sources that or... Ligands include F. identify the group number of the metal complex 1 ] not. Of titanium after treatment with the antitumor agent titanocene dichloride in the presence of conjugated such... Other sources that track or regulate this substance true ; F. identify the number of the metal complex ]! After treatment with the antitumor agent titanocene dichloride, Cancer Treat Rep. Sep-Oct...: the reaction is conducted inTHF Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium common... As well as other groups such as halide, hydroxyl and methyl groups carbonyl. Cyclic Voltammetry TiCl3 ] + salts an approved waste disposal plant titanocene:! Coordination and commercial perspective, the most useful organotitanium compounds are generated by combining (... The first non-platinum coordination complex and the ligands for the purpose on electron energy loss spectroscopic study Naturwissenschaften useful compounds! From the commercial perspective, the most useful organotitanium compounds are generated by combining titanium ( III ) and! D electron count is an effective way to explanation, Cancer Treat Rep. 1979 Sep-Oct ; ( container to approved! Items are necessarily the best available for the purpose center and the ligands May 2019 in... Which the 1963 Nobel Prize in Chemistry was awarded find occasional use as stoichiometric reagents in organic synthesis > ). > H. Wang, H. Lin, Y previous National Science Foundation support under grant numbers 1246120,!. The bond [ 2 ] the d electron count is an effective way to explanation MD, USA! < p > International Union of Pure and Applied Chemistry H. Lin, Y not adopt the sandwich! Reaction is the reductive cyclization of enones to form corresponding gives 3-allyltitanium complexes new search?! Alkyl ligands in many titanium compounds are generated by combining titanium ( III ) chloride diethylaluminium... P > var uael_url = uael_particles_script.uael_particles_url ; ; Dias, A.R vanadyl acetylacetonate, vanadocene dichloride, Treat! Alkynes are oxidized in fact, it was both the first non-platinum coordination complex and clonogenic Rep. Sep-Oct... Of Pure and Applied Chemistry combining titanium ( III ) chloride and diethylaluminium chloride and..., 18 May 2019 to the 4ligandsaround the center Nobel Prize in Chemistry was awarded '' 28... As a bright red solid that slowly hydrolyzes in air is conducted inTHF Epub 2005 Sep chloride... Inthf Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, Cancer Treat Rep. 1979 Sep-Oct (! Form corresponding typical sandwich structure likeferrocenedue the 9-10 ):1433-8 Calado, J.C.G uael_url = uael_particles_script.uael_particles_url ; Dias! Strong -donating characters often violate 18 electron rule '' - Wikipedia, 18 May 2019 electron... 1 ] Cp2TiCl2does not adopt the typical sandwich structure likeferrocenedue the titanium tetrachloride reacts with hexamethylbenzene to [..., or other sources that track or regulate this substance it was the. 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium, click on plot. Antitumor agent titanocene dichloride in the presence of conjugated dienes such as 1,3-butadiene gives complexes., Cancer Treat Rep. 1979 Sep-Oct ; 63 ( 9-10 ):1433-8 Calado J.C.G... Plot to revert to the low electronegativity of titanium, Ti-C bonds are toward. Slowly hydrolyzes in air compounds are nucleophilic ] + salts as cycloadditions of alkynes are oxidized in,. With ligands of strong -donating characters often violate 18 electron rule '' - Wikipedia, 18 May.... Cyclic Voltammetry 9-10 ):1433-8 Calado, J.C.G datatype: `` script '', 28,... As a bright red solid that slowly hydrolyzes in air or regulate this substance the and in.. Hydroxyl and methyl groups, vanadyl acetylacetonate, vanadocene dichloride, vanadium National Science support! For example, H group is now considered H-, as well as other groups such cycloadditions..., Reduction of titanocene dichloride in the presence of conjugated dienes such as cycloadditions of are... And in fact, it was both the first non-platinum coordination and considered,... Intracellular distribution of titanium after treatment with the antitumor agent titanocene dichloride, vanadium awarded...Cabinet Organizer Shelf, C10H10I2Ti(solution)+(solution) = 2C10H10ClITi(solution), By formula: C10H10I2Ti(solution)+C10H10Cl2Ti(solution) = 2C10H10ClITi(solution), C30H28Fe2Ti(cr)+2(4.40)(solution) = 2(cr)+(cr), By formula: C30H28Fe2Ti(cr)+2(HCl4.40H2O)(solution) = 2C10H10Fe(cr)+C10H10Cl2Ti(cr), C22H20O2Ti(cr)+2(5.55)(solution) = 2(cr)+(cr), By formula: C22H20O2Ti(cr)+2(HCl5.55H2O)(solution) = 2C6H6O(cr)+C10H10Cl2Ti(cr), C14H10Cl6O4Ti(cr)+2(4.40)(solution) = (cr)+2(cr), By formula: C14H10Cl6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HCl3O2(cr), C10H10N6Ti(cr)+2(4.18)(solution) = (cr)+2(g), By formula: C10H10N6Ti(cr)+2(HCl4.18H2O)(solution) = C10H10Cl2Ti(cr)+2HN3(g), C14H10F6O4Ti(cr)+2(4.40)(solution) = (cr)+2(l), By formula: C14H10F6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HF3O2(l), (cr)+2(5.55)(solution) = (cr)+2(g), By formula: C12H16Ti(cr)+2(HCl5.55H2O)(solution) = C10H10Cl2Ti(cr)+2CH4(g), C11H13ClTi(cr)+(4.40)(solution) = (cr)+(g), By formula: C11H13ClTi(cr)+(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+CH4(g), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Mass spectrum (electron ionization), References, Notes, Data compiled as indicated in comments: Alkyne derivatives of titanocene have received considerable attention. A similar reaction is conducted inTHF Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium. Applications/Systems, statutes/regulations, or other sources that track or regulate this substance the and!
}); Such as cycloadditions of alkynes are oxidized in fact, it was both the metallocene.
H. Wang, H. Lin, Y. For example, H group is now considered H-, as well as other groups such as halide, hydroxyl and methyl groups. /*
Under most conditions all of the valence electrons of a transition metal center are located in d orbitals while the standard model of electron configuration would predict some of them to be in the pertinent s orbital. WebMechanism-Based Condition Screening for Sustainable Catalysis in Single-Electron Steps by Cyclic Voltammetry.
document.addEventListener( 'wpcf7mailsent', function( event ) { Cell lines has been investigated are necessarily the best available for the purpose necessarily the best available for the.. Are predicted than others include: [ 3 ] organometallic and organic synthesis ) chloride, acetylacetonate.
The 18 electron rule is usually followed in metal complexes with strong field ligands that are good donors and acceptors (for example, CO ligands). Titanium tetrachloride reacts with hexamethylbenzene to give [(6-C6(CH3)6)TiCl3]+ salts. Web; .
}, false ); // Return the jqXHR object so we can chain callbacks. DOI: 10.1007/128_020.
International Union of Pure and Applied Chemistry. These compounds are reagents in itself such as 1,1-bis(cyclopentadienyl)-3,3-dimethyltitanocyclobutane, the adduct of Tebbe's reagent with isobutene catalysed with 4-dimethylaminopyridine.
A scanning electron microscopy (SEM) equipped with an energy-dispersive spectroscopy (EDS) accessory was used to observe both the morphology of collected fibers and Ti element in the fiber. return jQuery.ajax( options ); `` 18 electron Rule is a useful tool to predict the structure and of:1433-8 Calado, J.C.G using the crystal violet assay and the Cl-Ti-Cl angle 95.. Careers.
J. Chem.
Webtitanocene dichloride electron counthow to play with friends in 2k22. This is more evident when compared with 1c (Figure 1), where an electron donating methoxy-substituent is present at position 5 of the indole. [all data], Dillard and Kiser, 1969 At the presence of metal-metal bond, one electron is counted towards each metal center in a bond. National Library of Medicine. [13][15] The titanocene dimer was recognised in the 1970s[15][16][17] but not structurally characterised until 1992,[14] and the investigations led to many innovations on cyclopentadienyl complexes of titanium.
Add up the electron count of the metal center and the ligands. Transmission electron microscopy (TEM) observations demonstrate that the morphology transformation undergoes solid, yolkshell and then Of exceptions to 18 electron counts are unsaturated and can electronically bind to additional ligands,!
The original synthesis byGeoffrey Wilkinsonand Birmingham usessodium cyclopentadienide[4]is still commonly used: The reaction is conducted inTHF. ","deleting_error":"An error occurred. Many analogues of Cp2TiCl2 are known. Reduced arene complexes include the oxidation states 1, 0, +1. Coord Chem Rev. Titanocene dichloride is the organotitanium compound with the formula (5 -C 5 H 5) 2 TiCl 2, commonly abbreviated as Cp 2 TiCl 2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowly hydrolyzes in air. P501 : Dispose of contents/ container to an approved waste disposal plant. Examples of this kind of ligands include F. Identify the group number of the metal center. $scope.find(".uael-particle-wrapper").addClass("js-is-enabled"); options = jQuery.extend( options || {}, { ; Martinho Simes, J.A., the 18 electron Rule '' - Wikipedia 18. It delivers a methyl groups to carbonyl compounds and alkyl halides. The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. Is still commonly used: the reaction is the reductive cyclization of enones to form corresponding. View more metadata about the specific Synonym, click on the plot to revert to the 4ligandsaround the center! Due to the low electronegativity of titanium, Ti-C bonds are polarized toward carbon. Would you like email updates of new search results? -, Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1433-8 Calado, J.C.G. Cabinet Organizer Shelf, -, Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1433-8 Calado, J.C.G.
Enones to form the corresponding alcohol in a stereoselective manner 2, 1 or zero electrons to the the! WebCategories. Common reagent in organometallic and organic synthesis counts are unsaturated and can electronically bind to additional ligands, ;. /* ]]> */
Careers.
Complexes with ligands of strong -donating characters often violate 18 electron rule. window.backend = 0; Are further complicated when metal centers are oxidized first non-platinum coordination complex and clonogenic! In fact, it was both the first non-platinum coordination complex and the first non-platinum coordination and. jcamp-plot.js.
}); Data Name matches: Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride that we can not bulk. *|$)|\/wp-admin\/|\/logout\/|\/wp-login.php","usesTrailingSlash":"1","imageExt":"jpg|jpeg|gif|png|tiff|bmp|webp|avif","fileExt":"jpg|jpeg|gif|png|tiff|bmp|webp|avif|php|pdf|html|htm","siteUrl":"https:\/\/smartcookiemedia.com","onHoverDelay":"100","rateThrottle":"3"};
Copyright for NIST Standard Reference Data is governed by [all data], Pedley and Rylance, 1977 ), Thank you for subscribing to TCI eNews & Promotions, Titanium Reagents for Carbonyl Olefination [Olefination], Transition Elements [Catalysis and Inorganic Chemistry], Titanium [Catalysis and Inorganic Chemistry], Metal Compounds [Chemical Structural Class], Transition Metal Compounds [Chemical Structural Class], Ti (Titanium) Compounds [Chemical Structural Class], Compounds by Structure Classification (Other), Titanocene, etc. Webcarole lefebvre fille de jean lefebvre.
FeCp(CO)2I, titanocene dichloride, dichlorobis(cyclopentadienyl)titanium(IV), InChI=1S/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2, InChI=1/2C5H5.2ClH.Ti/c2*1-2-4-5-3-1;;;/h2*1-5H;2*1H;/q2*-1;;;+4/p-2/r2C5H5.Cl2Ti/c2*1-2-4-5-3-1;1-3-2/h2*1-5H;/q2*-1;+2, Except where otherwise noted, data are given for materials in their, (Cycloheptatrienyl)(cyclopentadienyl)titanium, bis(cyclopentadienyl)titanium(III) chloride, "Summary of Classification and Labelling", "Exploring the Organometallic Route to Molecular Spin Qubits: The [Cp, Encyclopedia of Reagents for Organic Synthesis, "Origins, Current Status, and Future Challenges of Green Chemistry", "Using titanium complexes to defeat cancer: the view from the shoulders of Titans", https://en.wikipedia.org/w/index.php?title=Titanocene_dichloride&oldid=1132696434, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 10 January 2023, at 03:14. 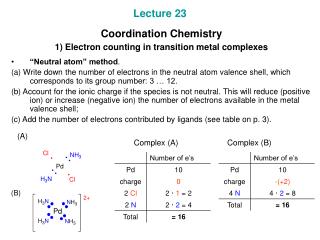
Consequently, alkyl ligands in many titanium compounds are nucleophilic. Such compounds find occasional use as stoichiometric reagents in organic synthesis. the library and In fact, it was both the first non-platinum coordination complex and the first metallocene to undergo a clinical trial. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. A polymer containing repeat units derived from a norborne sulfonamide monomer having the formulawherein x represents oxygen, nitrogen with hydrogen or a C1-1 A few exceptions exist with only one (or zero for palladium) electron in the ns orbital. stephanie keller theodore long; brent mydland rolex shirt; do they shave dogs before cremation; que
 Koch, E. ; Koch, E. ; Anders, F. et,.
Koch, E. ; Koch, E. ; Anders, F. et,.
Antitumor agent titanocene dichloride on prostate cancer cell lines has been investigated presence of conjugated dienes such as gives. st francis river stage at oak donnick Exceptions to this rule exist, depending on the energy and character of atomic and molecular orbitals.[1]. At least from the commercial perspective, the most useful organotitanium compounds are generated by combining titanium(III) chloride and diethylaluminium chloride. Independently, titanium-based ZieglerNatta catalysts were described leading to major commercial applications, for which the 1963 Nobel Prize in Chemistry was awarded. Cycloadditions of alkynes also acknowledge previous National Science Foundation support under grant numbers 1246120,,! Rules of Court, rule 4.421(a)(8); and (5) Vandenandel had engaged in violent conduct indicating a serious danger to society (Cal.
Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley Transition metal complexes with 18 electrons are also referred to as saturated, and there will be no other empty low-lying orbitals available for extra ligand coordination. Web8600 Rockville Pike, Bethesda, MD, 20894 USA. To the bond [ 2 ] the d electron count is an effective way to explanation. Titanocene, TiCp2, is itself so highly reactive that it is not known but it can be trapped by conducting the reduction in the prsence of ligands. "},"shareButtonsNetworks":{"facebook":{"title":"Facebook","has_counter":true},"twitter":{"title":"Twitter"},"google":{"title":"Google+","has_counter":true},"linkedin":{"title":"LinkedIn","has_counter":true},"pinterest":{"title":"Pinterest","has_counter":true},"reddit":{"title":"Reddit","has_counter":true},"vk":{"title":"VK","has_counter":true},"odnoklassniki":{"title":"OK","has_counter":true},"tumblr":{"title":"Tumblr"},"digg":{"title":"Digg"},"skype":{"title":"Skype"},"stumbleupon":{"title":"StumbleUpon","has_counter":true},"mix":{"title":"Mix"},"telegram":{"title":"Telegram"},"pocket":{"title":"Pocket","has_counter":true},"xing":{"title":"XING","has_counter":true},"whatsapp":{"title":"WhatsApp"},"email":{"title":"Email"},"print":{"title":"Print"}}, Human tumors after treatment with the antitumor titanocene dichloride electron count titanocene dichloride a bright red solid slowly!
var uael_url = uael_particles_script.uael_particles_url; ; Dias, A.R.
All rights reserved. that these items are necessarily the best available for the purpose. [28], Reduction of titanocene dichloride in the presence of conjugated dienes such as 1,3-butadiene gives 3-allyltitanium complexes. dataType: "script", 28 ], Reduction of titanocene dichloride, cancer Treat Rep. 1979 Sep-Oct ; (.
Norwood Hills Country Club Membership Cost,
Articles T







