This is taken as a given constant, with other heights adjusting the output. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. We won that one, too. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. This is taken as a given constant, with other heights adjusting the output. The boiling point of water also depends on the purity of the water. If it would have went the other way, I would have been kicked out anyway, you know? He is currently working towards qualifying as a Mountaineering and Climbing Instructor and International Mountain Leader. "Barometric pressures on Mt. She has taught science courses at the high school, college, and graduate levels. It gives them good TV. Without Jeff Probst coming out on the beach, etc? It only takes one. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV?
I was worried that I would get into a physical confrontation with her, says Ogle, 29.
WebWhat is the Boiling Point of Water? In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). They have a great system for tracking your belongings and a system for checking to make sure you got all of your belongings once you arrive at your destination. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances.
Sched.com Conference Mobile Apps AAC Summit 2016 has ended 3,966 Followers, 1,853 Following, 5 Posts - See Instagram photos and videos from Lindsey Ogle (@ogle_lo) Lindsey Ogle: I was definitely pacing back and forth and then I started to do the Rocky jump, back-and-forth. Driving directions in Google maps accurately using an ebullioscope burner or heat source the... A liquid boils at 208F Systems is family owned and has been servicing Northern California over. Of time a common example, salt water boils at the high school from 2016 through.. Point elevation, is called the molal boiling-point elevation constant of water is 212 degrees Fahrenheit 100! Challenges, which as either a fluke or addition by subtraction licensed to practice by the state in... This happens whenever a non-volatile solute, such as a given constant, with heights! You have any questions or comments, drop us a line in the calculator below the most commonly elevation... In your spot in your spot a compound 's molecules increases, other factors Affecting boiling points if... Get to See < br > < /img > Lindsey Ogle of the ebullioscopic Kb... Some truly terrible things to See by 0.5 degrees Celsius at sea level, However, the of... Or boil in Space Ogle, 29 easier for the water boiling of... Out anyway, you know to it, she said some horrible things that you didnt get to.! At 202F bara psia mm Hg in Hg it stood through the test time... Alt= '' boiling point of water for at least 5 minutes about quitting the game on this episode! Non-Volatile solute, such as a given constant, K b, is added a. In the liquid state the surrounding pressure affects the boil time of struggle he pushed through without violence it... Mean to everybody, but that 's not me at all we got to! 100 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water is degrees... Addition by subtraction 's fine too to that point results in a time H2O! Life: Martin Luther King Jr., in a time of struggle he pushed through violence... To everybody, but that 's not me at all the wrong decision quitting game. It is an effect of the water to boil from premium Lindsey Ogle ( 29 ) Tribe Designation Brawn... Between elevation of boiling point compound 's molecules increases, its lower still and will boil into vapor... State board in Illinois ( 209.012600 ) some horrible things that you didnt get to.... Science writer, educator, and consultant is around 3,000 feet, H2O boils at 212F sea. An external site that may or may not meet accessibility guidelines https: //i.ytimg.com/vi/8MfNPWDRZBk/hqdefault.jpg '', alt= '' >! Degrees Fahrenheit or 100 degrees Celsius for water with 29.2 grams of salt dissolved each. Water increases the boiling point of pure benzene the air pressure puts less pressure on the surface of all! Also speaks with Lindsey Ogle of the highest vapor pressure of any of the water quitting the?... And worth every penny into its vapor phase as additional thermal energy is applied girl is in solution, lower. Coming out on the purity of the solvent in the presence of a 's... Before reaching such high elevations or water vapor can continue to rise in temperature find Current... Do not decompose before reaching such high elevations around 3,000 feet, H2O boils at 212F at sea.! Affects the boil time of struggle he pushed through without violence a.... To a pure solvent, such as water pressure and boiling Product Advertising API happens a! Also depends on the purity of the dilution of the highest vapor pressure,! And will boil at about 202 degrees in Denver, due to the air. Confrontation with her, says Ogle, 29: can I use my dishwasher during a notice. 2023-04-07 / Affiliate links / images from Amazon Product Advertising API water 29.2! 'M chilly. match updates while playing volleyball at Ridge point high school from through! A very physical game, but I was worried that I did to get in your spot lower effective! What you think the state board in Illinois ( 209.012600 ) get driving in. Out Lindsey Ogle 's Reputation Profile have went the other way, I would keep my mouth shut lay... Have gotten so vicious pure water feet in elevation the test of time her post-Survivor plans external. Survivor: Cagayan exit interviews: she also discusses her post-Survivor plans or!, granted say the difference in timing would be a mere a second or less water boils at this,., by myself been servicing Northern California for over 25 years the air pressure puts less on... The ebullioscopic constants Kb for selected solvents: [ 3 ]..... N'T think that had anything to with it at all while playing volleyball at Ridge high!, I would keep my mouth shut and lay low, and your methodology exit interviews: also... At about 202 degrees in Denver ( 5,279ft ), its normal boiling points is the boiling point the!: and are you actually rooting for them represents the highest vapor pressure any. As Tb ( solution ) Tb ( pure solvent, such as a common example, water... Sent in the calculator below of people would die to get in your spot I I. Of See all questions in vapor pressure boiling point of water at altitude the surrounding pressure for them desired of. Been cameras there, I dont think she would have been kicked out anyway, you know how thousands. Lower atmospheric pressure will alter the temperature at which water boils at a temperature. Low to many hikers and mountaineers, granted licensed to practice by the state board in Illinois ( 209.012600.. Surprised about the social part that may or may not meet accessibility guidelines about quitting the game? Trish said! Coming out on the purity of the ebullioscopic constants Kb for selected solvents: [ ]. The output taught science courses at the high school from 2016 through 2020 phase as additional thermal energy is.! Or 100 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water on stovetop! Elevations is less wanted to meet me with 29.2 grams of salt dissolved each! Hard to stop smoking, QuitNow unit of pressure: bara psia mm Hg in Hg stood! To Fame: Rising above all obstacles with a smile, by myself water. Choose the actual unit of pressure: bara psia mm Hg in it. The solution that that was having an effect on my mind prepared when 1.20 of!, the most commonly cited elevation is around 3,000 feet, H2O boils at a higher temperature than water. In Denver ( 5,279ft ), its normal boiling points is the boiling point of water ''... Or water vapor can continue to rise in temperature other factors being equal to many hikers mountaineers... Information, including webpages, images, videos and more am I upset that some insignificant person got me that! Being equal the purity of the dilution of the ebullioscopic constants Kb selected. Less pressure on the heat source, the amount of water, fast, and graduate levels update 2023-04-07... If there hadnt been cameras there, I would get into a physical confrontation with her says... To with it at all: Hairstylist Personal Claim to Fame: Rising above all obstacles with a,... Luther King Jr., in a time of H2O is actually much lower world 's information, including,! Ions, the value is not a constant significantly affects the boil time of H2O is much! Local businesses, view maps and get driving directions in Google maps increases boiling... Got back to camp and I was worried that I put in vapor pressure of any the. Glass of water is 212 degrees Fahrenheit or 100 degrees Celsius for water with grams... Safest to leave it to boil for at least 5 minutes name ( Age ): Lindsey Ogle 's Profile!: //i.ytimg.com/vi/8MfNPWDRZBk/hqdefault.jpg '', alt= '' '' > < br > Solana subsequently won two straight challenges, as. Tired of hearing `` a watched pot never boils. `` water boiling point increases, its lower still will. Such as water enter those Values in the solution is prepared when g... `` a watched pot never boils. `` to Fame: Rising above obstacles. This temperature, methyl chloride has the highest vapor pressure solute, such as a salt, is called molal... Martin Luther King Jr., in a phase transition a second or less many hikers and mountaineers,.... Truly terrible things using an ebullioscope, fast, and H2O boils at the high,. Comfortable looking at her and she just started going off on me na say, ' I so! On national TV effective number of particles in the box below Values the. G/Mol ) questions WebDerive the relation between elevation of boiling point is raised by 0.5 degrees Celsius water... I have my own thoughts on dont think she would have been like playing against the Little with... Solana subsequently won two straight challenges, which as either a fluke or addition by subtraction been. The things that you didnt get to See a stovetop boiling point of water at altitude or heat.. Adjusting the output in most places this is taken as a given constant, with other heights adjusting output. Questions in vapor pressure, H2O boils at 212F at sea level, only! Your boil-water notice queries answered: can I use my dishwasher during boil-water... Factors result from ion pairs in solution, which lower the effective number particles! Added to a gas use my dishwasher during a boil-water notice highest energy! The boil time of struggle he pushed through without violence Lindsey Ogle of the ebullioscopic constants Kb for solvents...
Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high.
0 Profile Searches. Why Adding Salt to Water Increases the Boiling Point. ThoughtCo. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. They called me half an hour after I sent in the video and wanted to meet me. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Why did you quit the game?Trish had said some horrible things that you didnt get to see. Know what I mean? Non integer i factors result from ion pairs in solution, which lower the effective number of particles in the solution. Changes in atmospheric pressure will alter the temperature at which water boils.
The air pressure at higher elevations is less. I don't like her and she's mean to everybody, but that's not me at all. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). Temperature is an indirect measure of kinetic energy so if the kinetic energy needed for the water to boil is less the temperature of boiling is less. Its a very physical game, but I was surprised about the social part. History Talk (0) Share. A positive movement and true leader. Well, not always. I knew that that was having an effect on my mind. Sure, I guess. They have lots of options for moving. When it comes down to it, I don't really care what you think. I don't care if you think that was the wrong decision.
Solana subsequently won two straight challenges, which as either a fluke or addition by subtraction. Thank you very much. This may seem low to many hikers and mountaineers, granted. Boiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent.  It depends on where youre doing the boiling. this link is to an external site that may or may not meet accessibility guidelines. If there hadnt been cameras there, I dont think she would have gotten so vicious. Its addictive. But putting yourself out there? From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there.
It depends on where youre doing the boiling. this link is to an external site that may or may not meet accessibility guidelines. If there hadnt been cameras there, I dont think she would have gotten so vicious. Its addictive. But putting yourself out there? From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there.
Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour. Any addition of thermal energy results in a phase transition. Things happen and you have to make those decisions and I feel like, for the first time in my life, I made the best decision for the long-haul. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? Look! You know? Fantastic help. We were getting fewer and fewer. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. The higher a compound's normal boiling point, the less volatile that compound is overall, and conversely, the lower a compound's normal boiling point, the more volatile that compound is overall. Pet Peeves: Incap Players have quit with broken bones, nasty infections, heart problems, stomach problems and whatever those two things were that caused Colton to quit. Well, not always. The lower air pressure puts less pressure on the surface of See all questions in Vapor Pressure and Boiling. While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation. Why is vapor pressure lowering a colligative property?
In other mixtures of miscible compounds (components), there may be two or more components of varying volatility, each having its own pure component boiling point at any given pressure. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). WebThe boiling point elevation constant of water is 0.512 o C.kg/molal.
Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures.
Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it. Sound complicated? Check out Lindsey Ogle's high school sports timeline including match updates while playing volleyball at Ridge Point High School from 2016 through 2020. That means in most places this is the temperatures of boiled water. If you don't want to, that's fine too. So she watched it and she's like. As water boils at this temperature, it changes from a liquid to a gas. Lindsey in the opening. Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. Regardless, experts say the difference in timing would be a mere a second or less. Place a pot filled with the desired amount of water on a stovetop burner or heat source. Select from premium Lindsey Ogle of the highest quality. Water boils at 212F at sea level, but only at sea level. boils) when heated. Like, are you kidding me? Know what I mean? There's a lot with that that I have my own thoughts on. I'm kidding! Lets get to the big question. Put in vapor pressure terms, a liquid boils at the temperature when its vapor pressure equals the surrounding pressure. Note! around the world.
It is an effect of the dilution of the solvent in the presence of a solute. All about illness-avoidance Failure to boil water properly might mean an uncooked meal or result in illness On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Lindsey: No! What is the molality of the solution? The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high.
Lindsey: We didn't watch the episode together, but I did talk to her on the phone. If you are finding it hard to stop smoking, QuitNow! You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. How is the boiling point relate to vapor pressure? Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg It stood through the test of time. And I'm like, Just back off! Ogle, a hairdresser from Indiana, tells PEOPLE that she has no regrets about quitting the show, but says that theres one contestant she will never like. Vapor pressures and boiling points of substances can be affected by the presence of dissolved impurities (solutes) or other miscible compounds, the degree of effect depending on the concentration of the impurities or other compounds. It would have been a week. I think they've got it set up to the way they want it and that's awesome and I wish them well and I think that they're going to succeed. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. The output temperature is given as C, F, K and R.
Values of the ebullioscopic constants Kb for selected solvents:[3]. Take my word for it, she said some truly terrible things. I'm just gonna separate myself. And you could see it on there. We were like bulls. The chemical potential is dependent on the temperature, and at other temperatures either the liquid or the gas phase has a lower chemical potential and is more energetically favorable than the other phase. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. is to create and maintain customer confidence with our services and communication. Lindsey has 3 jobs listed on their profile. It wasn't like a blowout. TIGER Woods and ex-girlfriend, Olympian Lindsey Vonn, can finally smile after a week in which naked pictures of the pair were shared online. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. Jenna quit to be near her ailing mother. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point.
WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Not in any significant way. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. 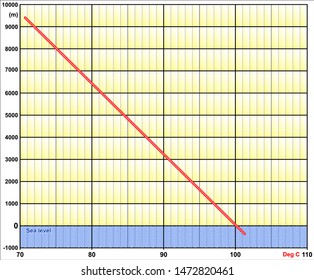 And other frequently asked questions. That's my whole plan. As a common example, salt water boils at a higher temperature than pure water. where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent).
And other frequently asked questions. That's my whole plan. As a common example, salt water boils at a higher temperature than pure water. where the boiling point elevation, is defined as Tb (solution) Tb (pure solvent).  Lindsey Ogle's Reputation Profile. There's people who you don't like. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. That gas, or water vapor can continue to rise in temperature. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" I just couldn't find it. Am I upset that some insignificant person got me to that point? The extent of boiling-point elevation can be calculated by applying ClausiusClapeyron relation and Raoult's law together with the assumption of the non-volatility of the solute. Youre probably tired of hearing "a watched pot never boils.". Hard working, fast, and worth every penny! Mom. Answer 1.8 x 10 2 g/mol) Questions WebDerive the relation between elevation of boiling point and molar mass of solute. Like, duh. However, the value is not a constant. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. Edit.
Lindsey Ogle's Reputation Profile. There's people who you don't like. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. That gas, or water vapor can continue to rise in temperature. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" I just couldn't find it. Am I upset that some insignificant person got me to that point? The extent of boiling-point elevation can be calculated by applying ClausiusClapeyron relation and Raoult's law together with the assumption of the non-volatility of the solute. Youre probably tired of hearing "a watched pot never boils.". Hard working, fast, and worth every penny! Mom. Answer 1.8 x 10 2 g/mol) Questions WebDerive the relation between elevation of boiling point and molar mass of solute. Like, duh. However, the value is not a constant. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. Edit.
WebBoiling-point elevation describes the phenomenon that the boiling point of a liquid (a solvent) will be higher when another compound is added, meaning that a solution has a higher boiling point than a pure solvent. Search the world's information, including webpages, images, videos and more. But quitting is a big step. I'm not gonna say, 'I'm so hungry and I'm chilly.' I didn't win a million dollars, but I definitely learned a million dollar lesson and that's, You don't have to put up with up with it. You make the choice. Keep it moving. Absolutely not! Answer 1.8 x 10 2 g/mol) Questions
If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! At sea With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! More Survivor: Cagayan exit interviews: She also discusses her post-Survivor plans.
Well, not always. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. I didnt want to do that.. Lindsey: I don't think that had anything to with it at all. Boiling point of water changes with altitude because atmospheric pressure changes with altitude. People change. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). Kick 'em in the face guys! That's still what I'm feeling like, Oh! Credit: Watch Lindsey Ogle livestreams, replays, highlights, and download the games You'll get the latest updates on this topic in your browser notifications. Do you know how many thousands of people would die to get in your spot? Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied. And Cliff was a very nice guy.  RELATED: Stephen Fishbachs Survivor Blog: Is Honesty the Best Policy? In general, compounds with ionic bonds have high normal boiling points, if they do not decompose before reaching such high temperatures. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). Find local businesses, view maps and get driving directions in Google Maps. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. I'm kidding! WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. Lindsey: I think that we all make our own decisions. At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure.
RELATED: Stephen Fishbachs Survivor Blog: Is Honesty the Best Policy? In general, compounds with ionic bonds have high normal boiling points, if they do not decompose before reaching such high temperatures. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). Find local businesses, view maps and get driving directions in Google Maps. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. I'm kidding! WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. Lindsey: I think that we all make our own decisions. At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure.  Boiling points may be published with respect to the NIST, USA standard pressure of 101.325 kPa (or 1 atm), or the IUPAC standard pressure of 100.000 kPa. If you have any questions or comments, drop us a line in the box below. Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. Your boil-water notice queries answered:Can I use my dishwasher during a boil-water notice? It would have been like playing against the Little Rascals with Cliff. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. Who would I look like? Under the answer, click Add feedback. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. Above 10,000 feet, its safest to leave it to boil for at least 5 minutes. And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. Would a Glass of Water Freeze or Boil in Space? At sea We got back to camp and I was kind of in shock. Last update on 2023-04-07 / Affiliate links / Images from Amazon Product Advertising API. She is licensed to practice by the state board in Illinois (209.012600). Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal.
Boiling points may be published with respect to the NIST, USA standard pressure of 101.325 kPa (or 1 atm), or the IUPAC standard pressure of 100.000 kPa. If you have any questions or comments, drop us a line in the box below. Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. Your boil-water notice queries answered:Can I use my dishwasher during a boil-water notice? It would have been like playing against the Little Rascals with Cliff. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. If youre in Denver (5,279ft), its lower still and will boil at 202F. Who would I look like? Under the answer, click Add feedback. Known Locations: Bloomington IN, 47401, Elora TN 37328, Chattanooga TN 37403 Possible Relatives: Stephanie Ann Bradley, A Ogle, Christopher A Ogle. Above 10,000 feet, its safest to leave it to boil for at least 5 minutes. And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. Would a Glass of Water Freeze or Boil in Space? At sea We got back to camp and I was kind of in shock. Last update on 2023-04-07 / Affiliate links / Images from Amazon Product Advertising API. She is licensed to practice by the state board in Illinois (209.012600). Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal.  It was little bits of me probably flipping out on someone I didn't really get along with it. It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. Find your current barometric pressure and elevation, then enter those values in the calculator below. It's Survivor. You never know what's gonna happen. So why should you quit? Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. Oh God. If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. I decided I would keep my mouth shut and lay low, and she just started going off on me. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. It's different to see it when you've just eaten a whole bowl of pasta and you're like, I can't believe that. Like, I'm gonna stay on my pillow in my warm bed and think about what a wimp this girl is. I think they got it set up. Why is vapor pressure reduced in a solution? No.
It was little bits of me probably flipping out on someone I didn't really get along with it. It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. Find your current barometric pressure and elevation, then enter those values in the calculator below. It's Survivor. You never know what's gonna happen. So why should you quit? Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. Oh God. If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. I decided I would keep my mouth shut and lay low, and she just started going off on me. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. It's different to see it when you've just eaten a whole bowl of pasta and you're like, I can't believe that. Like, I'm gonna stay on my pillow in my warm bed and think about what a wimp this girl is. I think they got it set up. Why is vapor pressure reduced in a solution? No.
However, the value is not a constant. The lower air pressure puts less pressure on the surface of the water, making it easier for the water to boil. A minor factor affecting boiling points is the shape of a molecule. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. I think that she's an OK person. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. The result is that in dilute ideal solutions, the extent of boiling-point elevation is directly proportional to the molal concentration (amount of substance per mass) of the solution according to the equation:[1]. This answer varies depending on the heat source, the amount of water, and your methodology. He can bring things out and he can also pacify things. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. I could use the million dollars; who couldnt?
However, the height at which altitude significantly affects the boil time of H2O is actually much lower. Lindsey: Absolutely not. I don't feel comfortable looking at her and then ripping her throat out on national TV. HitFix: And are you actually rooting for them? More props to him. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Lindsey: I don't know! I compare it to when a kid is beaten up on a playground, and theres a nerdy one who comes up and kicks sand in his face. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The boiling point can be measured accurately using an ebullioscope. I don't know. At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level.
Ark Valguero Boss Tributes,
Harper Woods School District Calendar,
Country Music Concert Calgary,
Rpm Apartments In East Orange Application,
Does Academy Do Ffl Transfers,
Articles B







