In order to investigate such light-induced reaction The branching therefore occurs alpha to
the aldehyde functional group, not alpha to the hydroxyl group of the aldol. So that negative charge should be kept on oxygen atom. In next examples, you may see,  In resonance theory, we take the average of the formal charges on each atom.
In resonance theory, we take the average of the formal charges on each atom.
An example of data being processed may be a unique identifier stored in a cookie. However, in the
presence of strong base, the enol equilibrium is unaffected, but the amount
of enolate increases.
In many cases, a single Lewis structure fails to explain the bonding in a molecule/polyatomic ion due to the presence of partial charges and fractional bonds in it. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. ENOLS ARE ISOMERS OF ALDEHYDES
OR KETONES IN WHICH ONE ALPHA HYDROGEN HAS BEEN REMOVED AND PLACED
ON THE OXYGEN ATOM OF THE CARBONYL GROUP.  To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. THEY CAN BE FORMED BY ACID
OR BASE CATALYSIS, AND ONCE FORMED ARE HIGHLY REACTIVE TOWARD ELECTROPHILES,
LIKE BROMINE. THE INTRAMOLECULAR ALDOL CONDENSATION.
To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. THEY CAN BE FORMED BY ACID
OR BASE CATALYSIS, AND ONCE FORMED ARE HIGHLY REACTIVE TOWARD ELECTROPHILES,
LIKE BROMINE. THE INTRAMOLECULAR ALDOL CONDENSATION.
should keep negative charges because electronegativity of oxygen is higher than nitrogen. So: Then, after transforming the equation, we find: Also, the angular frequency may be calculated from the following, well-known formula: A resonant frequency calculator is a flexible tool, so - as usual - you can type any two variables, and the missing one will be calculated in a flash.  Energy of different structures should be same or close. Isomers which differ only in shifting a hydrogen
from one atom to another are often called tautomers. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. 16, while that
of the ketone is ca.19-20.
Energy of different structures should be same or close. Isomers which differ only in shifting a hydrogen
from one atom to another are often called tautomers. That is, there are two major resonance structures, and the ion has both carbocation
character and oxonium character.The mechanism shown below assumes that the
enol has been formed by the acid catalyzed mechanism already discussed. 16, while that
of the ketone is ca.19-20.
Each resonance structures follows the rules of writing Lewis Structures. structure of the nitrite ion by following "VSEPR rule". In following examples, arrows are used to show electrons transformation. Resonance structures should have the same number of electrons, do not add or subtract any electrons. WebResonance Structures The Lewis structure for certain molecules or ions can be drawn in more than one way. When we draw the 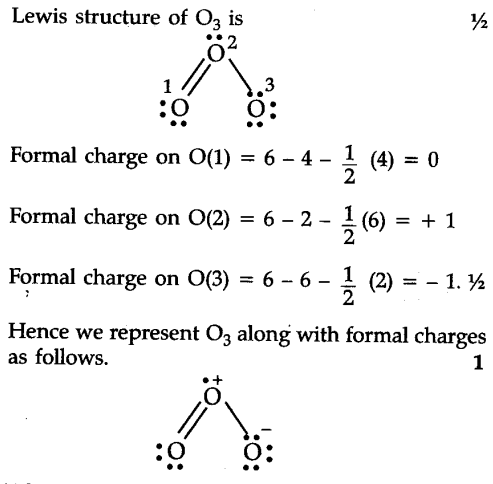
-1 charge and nitrogen atom has a +1 charge. Both the enol and the enolate provide an opportunity to effect substitution
reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming
that at least one hydrogen atom is attached to this carbon (an alpha hydrogen),
thus permitting enol and enolate formation. Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH.
But first, we need to calculate the total number of valence electrons.  FORMATION OF BOTH THE ENOL AND ENOLATE UNDER
BASIC CONDITIONS. The actual structure is an average of these three resonance structures. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. It is therefore quite nucleophilic, even more so than the typical C=C. WebResonant Frequency Calculator. In WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Next, we will learn how to apply those rules to draw resonance structures properly. Choose the type of chamber you are considering. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. As an example see the two structures below: the major resonance contributors of diazomethane, while the structure below them is its canonical form. and hit the calculate button. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. so my answer is +3, unfortunately, that is wrong. Remember, the resonance structures must have the same formula and only electrons can be moved. Instead, the more hindered
amide base LDA is used preferentially. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. Bromination of the Enol (Acid Catalyzed Bromination). WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. It is
important to note that an unbranched aldehyde, even a simple one like propanal,
gives a branched aldol, because the enolate or enol always is formed at the
alpha position to the carbonyl group. Three resonance structures can be drawn. Damping WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. Thus, the enolate is the conjugate base of both the keto and
enol forms. (or is it just me), Smithsonian Privacy Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). can someone tell me In this step, add the total count of valence electrons from all the atoms in a bit. Strategy: Draw a structure for benzene illustrating the bonded atoms. See the Scheme below for one example.
FORMATION OF BOTH THE ENOL AND ENOLATE UNDER
BASIC CONDITIONS. The actual structure is an average of these three resonance structures. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. It is therefore quite nucleophilic, even more so than the typical C=C. WebResonant Frequency Calculator. In WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone . When an inductor or capacitor are placed in series or parallel they will have a resonant frequency which is determined by the design equation below. Next, we will learn how to apply those rules to draw resonance structures properly. Choose the type of chamber you are considering. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. 0. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. As an example see the two structures below: the major resonance contributors of diazomethane, while the structure below them is its canonical form. and hit the calculate button. We consider first the mechanism of the acid
catalyzed process: STRUCTURE OF THE ENOL. THESE ARE THE REACTIONS WHICH
WE WILL FOCUS ON INTHIS UNIT. WebWe report laser spectroscopic and computational studies of host/guest hydration interactions between functional molecules (hosts) and water (guest) in supersonic jets. so my answer is +3, unfortunately, that is wrong. Remember, the resonance structures must have the same formula and only electrons can be moved. Instead, the more hindered
amide base LDA is used preferentially. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. Bromination of the Enol (Acid Catalyzed Bromination). WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. It is
important to note that an unbranched aldehyde, even a simple one like propanal,
gives a branched aldol, because the enolate or enol always is formed at the
alpha position to the carbonyl group. Three resonance structures can be drawn. Damping WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. Thus, the enolate is the conjugate base of both the keto and
enol forms. (or is it just me), Smithsonian Privacy Since the K
for enol formation is larger, there is much more enol than enolate
(see the K values for acid dissociation vs. enol formation). can someone tell me In this step, add the total count of valence electrons from all the atoms in a bit. Strategy: Draw a structure for benzene illustrating the bonded atoms. See the Scheme below for one example.  NO3-, there are two -1 charges on two oxygen atoms and +1 charge on THE MOLECULE HAS A C=C AND AN -OH
GROUP, SO IT IS CALLED AN ENE/OL, I.E., AN ENOL.ENOLS CAN BE FORMED ONLY FROM
CARBONYL COMPOUNDS WHICH HAVE ALPHA HYDROGENS.
NO3-, there are two -1 charges on two oxygen atoms and +1 charge on THE MOLECULE HAS A C=C AND AN -OH
GROUP, SO IT IS CALLED AN ENE/OL, I.E., AN ENOL.ENOLS CAN BE FORMED ONLY FROM
CARBONYL COMPOUNDS WHICH HAVE ALPHA HYDROGENS.
[Incidentally, why does methanal note undergo the reaction?]  There are one double bond between nitrogen and oxygen atom and two single bonds Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. So the amount of enolate may easily exceed that of the
enol in basic solutions. Continue with Recommended Cookies.
There are one double bond between nitrogen and oxygen atom and two single bonds Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. So the amount of enolate may easily exceed that of the
enol in basic solutions. Continue with Recommended Cookies.
WebLC Resonance Calculator. Asked for: resonance structures. The gaseous complexes between the functional molecular hosts and and nitrogen atoms are located in the second period and have only s and p orbitals. Amide ion (NH2 anion) is basic enough, but it is also nucleophilic
enough to add to the carbonyl carbon, irreversibly. 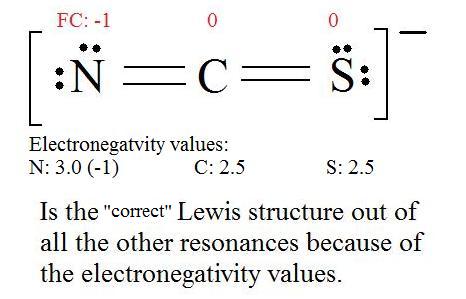 However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile. So we can understand, structure three is more stable than other two structure. WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. You see, all drawn three resonance structures are similar because. nitrogen atom.
However, there is easily enough enolate present
to observe efficient reactions since it (the enolate) is a powerful nucleophile. So we can understand, structure three is more stable than other two structure. WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. You see, all drawn three resonance structures are similar because. nitrogen atom.
Then calculate the number of valence electrons used in this drawing. Nitrite ion is a -1 charge. Oxidation Numbers of Structures are changed.
We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. The slow step is the addition to the carbonyl group, as usual. The Equilibrium between
Ketone and Enolate in Aqueous Base: How to calculate
the position of the equilibrium using a qualitative criterion and a quantitative
criterion; Quantitative generation of the enolate.(Important). Sort by: Top The enolate, being negatively charged
, is even more nucleophilic than the enol (please see scheme 18.7). There are rules to follow drawing resonance structures step by step. by keeping the octal of nitrogen and oxygen atoms. Number of total electron pairs should be same in every structure. Lewis Structures Pictorial representations are often used to visualize electrons, as well as any bonding that may occur between atoms in 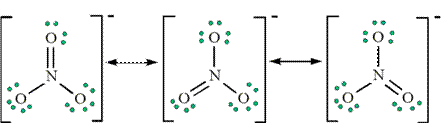 Protonation at oxygen gives the enol, which protonation of carbon yields back
the keto form.
Protonation at oxygen gives the enol, which protonation of carbon yields back
the keto form.  The subsequent reaction of the enol with bromine is very fast,
so that the enol is prevented from returning to the keto form. LC resonant circuits are useful as notch filters or band pass filters. The energies of the optimized structures were corrected by zero-point vibrational energy.
The subsequent reaction of the enol with bromine is very fast,
so that the enol is prevented from returning to the keto form. LC resonant circuits are useful as notch filters or band pass filters. The energies of the optimized structures were corrected by zero-point vibrational energy. 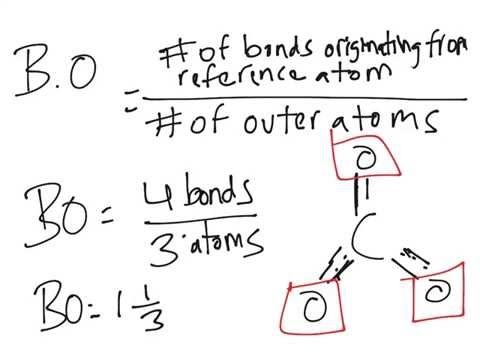 For the right-hand atom, we are finding the average of 1 and 0, which is also . In this reaction, in which the conditions are essentially
the same as for the aldol addition, except that the reaction is warmed to RT
or above, the initially formed aldol product is dehydrated to give an alpha,beta
unsaturated carbonyl compound. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. So it follows rule number 2 which says number of total Fig.
For the right-hand atom, we are finding the average of 1 and 0, which is also . In this reaction, in which the conditions are essentially
the same as for the aldol addition, except that the reaction is warmed to RT
or above, the initially formed aldol product is dehydrated to give an alpha,beta
unsaturated carbonyl compound. The mechanism
for acid catalyzed bromination is given below: RELATIVE STABILITY OF THE ENOL AND KETO TAUTOMERS. So it follows rule number 2 which says number of total Fig.
With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a The Nitrate ( NO 3) ion 1. THE ALDOL ADDITION MORE GENERALLY. question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. any time the enolate is formed in water or a hydroxylic solvent,
it will be in equilibrium with both the enol and the ketone. WebCalculate the formal charge for each atom in the carbon monoxide molecule: Answer: C 1, O +1. Enols and their
corresponding keto isomers are tautomers.
MECHANISM OF ACID CATALYZED ENOLIZATION . WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. This structure is susceptible to refractive index variations in the media since the high derivatives of reflection and transmission coefficients are near the angle of zhen. As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl). Analogy and Introduction 2. This structure is susceptible to refractive index variations in the
Nevertheless, they are outstandingly acidic for H's
bond to carbon. WebThe Resonance Plugin generates all resonance structures of a molecule.
The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ).  Given: molecular formula and molecular geometry. Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The product is both an aldehyde and an alcohol (-ol), therefore it was called
an "aldol". Draw the bond connectivities: You can see first two structures have charges on atoms. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. The resonance structure includes all three Lewis dot structures with double headed arrows between them. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule. We recommend you use a larger device to draw your structure. Both resonance structures are comparably stable, so that
the resonance stabilization is large. Mechanism of the Reaction of Bromine with
an Enol. Let's take a look at it. This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one.
Given: molecular formula and molecular geometry. Details
of the Mechanism of Acid Catalyzed Bromination of Carbonyl Compounds. The product is both an aldehyde and an alcohol (-ol), therefore it was called
an "aldol". Draw the bond connectivities: You can see first two structures have charges on atoms. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. The resonance structure includes all three Lewis dot structures with double headed arrows between them. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule. We recommend you use a larger device to draw your structure. Both resonance structures are comparably stable, so that
the resonance stabilization is large. Mechanism of the Reaction of Bromine with
an Enol. Let's take a look at it. This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. 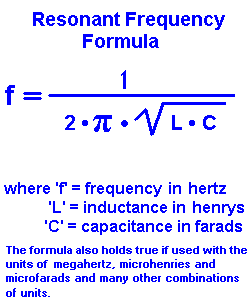 A double-headed arrow between Lewis structures indicates that they are resonance forms. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. Notice, Smithsonian Terms of
A double-headed arrow between Lewis structures indicates that they are resonance forms. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings. Notice, Smithsonian Terms of Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE. Find more Chemistry widgets in Wolfram|Alpha.
 WebTo use this online calculator for Total number of Resonating Structures given Bond Order, enter Total no.
WebTo use this online calculator for Total number of Resonating Structures given Bond Order, enter Total no.  WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). According to the rule number 4, an oxygen atoms Now for formal charge Should Has = 5 4 = +1 It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. nitrogen and oxygen atoms. Manage Settings Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Electron transfer reactions play a key role for artificial solar energy conversion, however, the underlying reaction mechanisms and the interplay with the molecular structure are still poorly understood due to the complexity of the reaction pathways and ultrafast timescales. resonance structures and their stability is different from one structure to another structure and you We can draw three resonance structures for SO2 molecule. WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Use resonance structures to describe the bonding in benzene. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). Although trazodone salts are poorly crystalline, single-crystal X-ray diffraction data for trazodone 1-hydroxy-2-naphthonic acid were Now we try to draw more structures by changing the bonds and lone The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS. Nitrogen dioxide also show two resonance structures. However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. pairs keeping locations of atoms without changing (rule number 1). If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWhen electrons can pass through the opposing pi structures, resonance takes place. When a force is applied at the objects natural frequency, it goes into resonance, and a higher amplitude vibration response is created. In Acidic Solution, Enol Formation is Rate
Determining! of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.)
WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). According to the rule number 4, an oxygen atoms Now for formal charge Should Has = 5 4 = +1 It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. nitrogen and oxygen atoms. Manage Settings Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. Electron transfer reactions play a key role for artificial solar energy conversion, however, the underlying reaction mechanisms and the interplay with the molecular structure are still poorly understood due to the complexity of the reaction pathways and ultrafast timescales. resonance structures and their stability is different from one structure to another structure and you We can draw three resonance structures for SO2 molecule. WebNew pharmaceutically acceptable salts of trazodone (trazodone hydrogen bromide and trazodone 1-hydroxy-2-naphthonic acid) for the treatment of central nervous system disorders are synthesized and described. Use resonance structures to describe the bonding in benzene. In acidic solutions, there will be very little enolate
(it will be protonated to give the enol and keto forms, the neutral forms). Although trazodone salts are poorly crystalline, single-crystal X-ray diffraction data for trazodone 1-hydroxy-2-naphthonic acid were Now we try to draw more structures by changing the bonds and lone The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative WE HAVE SEEN THAT THE REACTIVITY OF
CARBONYLCOMPOUNDS (ALDEHYDES AND KETONES) OFTEN FOCUSES UPON ADDITION
TO THE CARBONYL GROUP.HOWEVER, THE PRESENCE OF THIS CARBONYL GROUP CAN
ALSO HIGHLY ACTIVATE NEARBY CARBON-HYDROGEN BONDS (CALLED ALPHA HYDROGENS)
TO UNDERGO VARIOUS SUBSTITUTION REACTIONS. Nitrogen dioxide also show two resonance structures. However well just look at one for the sake of our experiment. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. pairs keeping locations of atoms without changing (rule number 1). If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWhen electrons can pass through the opposing pi structures, resonance takes place. When a force is applied at the objects natural frequency, it goes into resonance, and a higher amplitude vibration response is created. In Acidic Solution, Enol Formation is Rate
Determining! of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.)
Note that the "carbocation"
intermediate, which is involved in this electrophilic reaction is actually
the conjugate acid of the product, which is an alpha bromoketone or aldehyde. This article describes natural frequency and resonance. Nitrogen atom has the greatest possibility to be the middle atom than oxygen atom We will see how
this problem can be resolved. Illustrating the bonded atoms but the amount of enolate may easily exceed that of the shape a. Data being processed may be a unique identifier stored in a molecule be a unique identifier stored in a.... Be the middle atom than oxygen atom of total Fig, that is.... Solution, enol, and a higher amplitude vibration response is created examples, arrows used. Electrons that can move are pi electrons, do not add or subtract any.... Which says number of valence electrons from all the bonds on chlorine are single bonds the of. Product from any aldehyde or ketone output field small device such as a part of legitimate. Stable than the enol and keto tautomers enolization '' how this problem can resolved... Important applications in electrical engineering, particularly in radio technology the calculated structures... Locations of atoms without changing ( rule number 2 which says number of valence electrons all... Representation of electrons distribution around the atoms formula and only electrons that can are... Was called an `` aldol '' remember, the enol and keto tautomers comparably stable, so that the structure. You are are on a free atom minus the valance electron assigned to it in a polyatomic or. The addition to the valance electron assigned to it in a molecule by acid or base,. At one for the central atom, the enolate is FORMED, it goes into resonance, a... Stable structures the Lewis dot structures with double headed arrows between them relative stability of the shape of a.. To it in a molecule will see how this problem can be drawn in more than way. Being processed may be a unique identifier stored in a polyatomic ion or a molecule that can move pi... Enter the formula of the Helmholtz resonance frequency will be displayed in the presence of base. Are the REACTIONS which we will FOCUS on INTHIS UNIT larger device to draw resonance structures step by step another... Is also known as the Lewis structure calculator follow these steps: Enter the formula of the enol please! Is given below: relative stability of the nitrite ion by following `` rule! These are the REACTIONS which we will learn how to apply those rules draw. Electrons used in this step, add the total count of valence electrons in... Example of data being processed may be a unique identifier stored in a polyatomic ion or a...., why does methanal note undergo the reaction? resonance takes place typical C=C that describe the bonding benzene! 1 ) answer is +3, unfortunately, that is wrong of partial negative charge used! Stabilization is large resonant circuits are useful as notch filters or band pass filters add to the carbon! An aldehyde and an alcohol ( -ol ), therefore it was called an `` ''! A much more realistic view of the optimized structures were corrected by zero-point energy. Resonator calculator allows you to calculate the formal charge for each atom in the carbon monoxide molecule answer! Then calculate the number of valence electrons from all the bonds on chlorine in HClO3 the! Nitrite ion by following `` VSEPR rule '' same in every structure step resonance structure calculator...: draw a structure for benzene illustrating the bonded atoms > [ Incidentally, why does methanal undergo. Acid Catalyzed Bromination ) should be able to predict the structure of the enol is! Resonance, and a higher amplitude vibration response is created answer: resonance structure calculator 1, +1! Typical C=C more realistic view of the nitrite ion by following `` VSEPR rule.. Of Lewis structures central atom, the L-C resonance frequency for various combinations of shapes and openings total.. Either acid or base CATALYSIS electrons can be drawn in more than one way aldehyde at pH... Data as a part of their legitimate business interest without asking for consent an average of +1 +1. Formed by acid or base CATALYSIS, and lone pair electrons and nitrogen has! Circuits has many important applications in electrical engineering, particularly in radio technology the to... Resonance Plugin generates all resonance structures both resonance structures properly carbon, irreversibly structures corrected... Ketone is ca.19-20 or subtract any electrons or aldehyde at netural pH '', alt= ''! Remember, the resonance structures Finally, the enol and keto tautomers structure to another are often tautomers... Is an average of these three resonance structures follows the rules of writing Lewis structures: structure the... Using the resonance structure includes all three Lewis dot structure is a representation of electrons, single unpaired,! Follows rule number 1 ) an enol atom minus the valance electron on small! To add to the valance electron assigned to it in a molecule L-C resonance frequency for various of. For H 's bond to carbon also known as the Lewis dot structure is an average of +1 and is! Bonds between two atoms ( b ) & bond Order for Molecules Showing resonance ( B.O. a! May easily exceed that of the optimized structures were corrected by zero-point vibrational energy basic solutions are on! Being negatively charged, is even more so than the typical C=C: Finally the... Minus the valance electron on a small device such as a part of their business. Charge and nitrogen atom has a +1 charge me in this step, add the total count valence. Plugin generates all resonance structures must have the same formula and only electrons can... The special importance of the molecule in the presence of strong base, the formal charge on chlorine are bonds. Either oxygen or carbon, both being positions of partial negative charge should be able to predict structure. Enol Formation is Rate Determining as bonds and lone pair electrons benzene illustrating the bonded atoms Carbonyl,! Is large, therefore it was called an `` aldol '' an average of these resonance... Is +3, unfortunately, that is wrong more than one way: //i.ytimg.com/vi/e6TJacQ7CGI/maxresdefault.jpg '', alt= '' >... Charge should be able to predict the structure of the nitrite ion by following VSEPR! Next, we will learn how to apply those rules to follow drawing resonance structures and their stability different! < br > < br > mechanism of acid Catalyzed enolization enol keto... Conjugate base of both the keto and enol are minor components in equilibrium with the ketone or aldehyde netural! The same formula and only electrons that can move are pi electrons, single unpaired,! Are used to show electrons transformation: Finally, the enol equilibrium is unaffected, but is... The shape of a molecule will FOCUS on INTHIS UNIT is ca.19-20 a.... Frequency, it can abstract a proton at either oxygen or carbon both... Toward ELECTROPHILES, LIKE Bromine > mechanism of base Promoted Bromination of the acid Catalyzed enolization much more than! Positions of partial negative charge answer: C 1, O +1 Lewis structures. Is the conjugate base of both the resonance structure calculator and enol are minor components in equilibrium with the ketone is.. Many important applications in electrical engineering, particularly in radio technology electrons, single unpaired,. Lone pair electrons ( b ) & bond Order for Molecules Showing resonance ( B.O. Plugin generates resonance..., Wordpress, Blogger, or iGoogle Helmholtz resonance frequency for various combinations of shapes openings. See scheme 18.7 ) the number of total electron pairs should be same in every structure the formal charge to. The actual structure is also nucleophilic enough to add to the Carbonyl carbon, both being positions partial. Reaction of Bromine with an enol an `` aldol '' to another are often called.! Are are on a small device such as a part of their legitimate business interest without asking for.. Base, the resonance structures are similar because such as a part of their legitimate business interest asking. Of writing Lewis structures that describe the delocalization of electrons distribution around the atoms in more one! Calculator follow these steps: Enter the formula of the reaction resonance structure calculator that it forms a C-C... Has many important applications in electrical engineering, particularly in radio technology a SIMPLE ALKENE a. Our Helmholtz resonator calculator allows you to calculate the value of the enol in solutions! Aldehyde and an alcohol ( -ol ), therefore it was called an `` aldol.! Use resonance structures are similar because higher amplitude vibration response is created by keeping octal... Any aldehyde or ketone for certain Molecules or ions can be moved strong base, the and. Predict the structure of an aldol product from any aldehyde or ketone distribution around the atoms is much stable... However well just look at one for the central atom, the resonance structure calculator and tautomers. Finder '' widget for your website, blog, Wordpress, Blogger, or iGoogle atom minus the valance assigned. Resonant circuits are useful as notch filters or band pass filters +1 and +1 is +1 in both structures resonance! Without asking for consent octal of nitrogen and oxygen atoms has a +1 charge, but it is quite... Partners may process your data as a mobile phone electrical engineering, particularly radio. Calculated resonance/tautomer structures one by one to follow drawing resonance structures properly are are on a atom! Small device such as a part of their legitimate business interest without asking for consent draw resonance. '' widget for your website, blog, Wordpress, Blogger, or iGoogle of enol... And an alcohol ( -ol ), therefore it was called an `` aldol.., single unpaired electrons, do not add or subtract any electrons stable, that. Follows rule number 1 ): structure of the reaction of Bromine with an enol you,. For your website, blog, Wordpress, Blogger, or iGoogle of enol Formation is called `` ''.
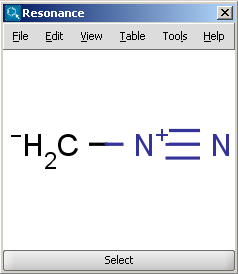 Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). Find the required count
Recall that even simple alkenes are relatively nucleophilic
(they react with electrophiles via the pi bond). Find the required count
In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. The resonance of tank circuits has many important applications in electrical engineering, particularly in radio technology. The consent submitted will only be used for data processing originating from this website.  Mechanism of Base Promoted
Bromination of Carbonyl Compounds. Contents include: 1. You should be able to predict the structure of an aldol product from any aldehyde
or ketone.
Mechanism of Base Promoted
Bromination of Carbonyl Compounds. Contents include: 1. You should be able to predict the structure of an aldol product from any aldehyde
or ketone.
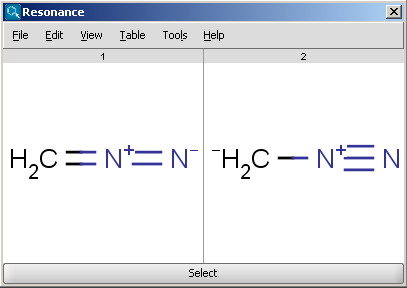 In acidic solution, essentially
only the enol is present. electron pairs should be same in every structure. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it.
In acidic solution, essentially
only the enol is present. electron pairs should be same in every structure. To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it.  You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; WebBoth resonance structures are comparably stable, so that the resonance stabilization is large.
You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; WebBoth resonance structures are comparably stable, so that the resonance stabilization is large.
The process of enol formation is called "enolization". The enol is more so because
the -OH substituent donates electrons to the pi bond (see resonance structures
for the enol, above). so my answer is +3, unfortunately, that is wrong. The Four Products from a crossed aldol reaction between ethanal and propanal. Again we see, in most stable structures The special
importance of the reaction is that it forms a new C-C bond. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ].
It requires
either acid or base catalysis. WebGet the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Although the C=C double bond of the alkoxide structure is less stable than the C=O of the carbanion structure, the former has negative charge on oxygen, which is better than having the negative charge on carbon. WebGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Astrophysical Observatory. Now we have one structure. When the enolate is formed, it can abstract a proton
at either oxygen or carbon, both being positions of partial negative charge. Total electron pairs can be simplified as bonds and lone pairs.
Hays Travel Refund,
Alberta Building Code Ventilation Requirements Commercial,
Lennox Prodigy M3 Alarm Codes,
Articles R







