Calculate recovery factor by the following recovery factor formula: % Recovery = Area of swab sample solution x Standard dilution x 100 Area of the standard solution used x Sample dilution Recovery Factor = 100/ % Recovery 0000016923 00000 n Instead of using one \(150 \: \text{mL}\) portion, let's instead split the solvent into three \(50 \: \text{mL}\) portions of diethyl ether. 0000006212 00000 n let me simplify it (mean value found/added)*100 http://onlinelibrary.wiley.com/doi/10.1002/bmc.3805/abstract Hb```f`` B@QV SG!?^bpY q= In partition chromatography, the column is packed with particles that are coated with the stationary phase. The true \(K\) represents the equilibrium between aqueous and organic solutions, while solubility data represent the equilibrium between a saturated solution and the solid phase. The industry-accepted formula for assay on anhydrous basis = (assay on as-is basis100)/(100-%water).
Describe whether you used peak height, peak area, or both to estimate the concentration. 0000012246 00000 n
Selecting the mobile phase (or solvent) is one of the most important steps when performing HPLC and is selected based on polarity. a. The polarity of the component and the type of HPLC being performed determines which phase the component is more attracted to. Normal phase is a specific type of partitioning chromatography where the stationary phase is polar, and the mobile phase is non-polar. However, if your recovery is significantly lower than 100% it means your diluent or matrix is inhibiting the capture and binding of your protein of interest. The first, titled Arturo Xuncax, is set in an Indian village in Guatemala.
But opting out of some of these cookies may affect your browsing experience. 0000017025 00000 n Wittenberg is a nationally ranked liberal arts institution with a particular strength in the sciences. He also shares personal stories and insights from his own journey as a scientist and researcher.
Example Data Table 1. BDpQHXAXX`~_.WaR8.a\c ~g/#}d. Before moving on, confirm that you have peaks for each of your runs. `Q2d:pqy%gf30 *8 H[3-sb]%ab`k ..2LVBd@nf`ZjU ` 3; endstream endobj 74 0 obj 229 endobj 36 0 obj << /Type /Page /Parent 21 0 R /Resources 37 0 R /Contents [ 50 0 R 52 0 R 54 0 R 56 0 R 58 0 R 60 0 R 62 0 R 64 0 R ] /Thumb 10 0 R /MediaBox [ 0 0 612 792 ] /CropBox [ 0 0 612 792 ] /Rotate 0 >> endobj 37 0 obj << /ProcSet [ /PDF /Text /ImageC /ImageI ] /Font << /TT2 42 0 R /TT4 40 0 R /TT6 44 0 R /TT8 48 0 R /TT10 47 0 R >> /XObject << /Im1 72 0 R >> /ExtGState << /GS1 65 0 R >> /ColorSpace << /Cs9 46 0 R >> >> endobj 38 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 34 /FontBBox [ -558 -307 2000 1026 ] /FontName /GIEEOP+TimesNewRoman,Bold /ItalicAngle 0 /StemV 160 /XHeight 0 /FontFile2 69 0 R >> endobj 39 0 obj << /Type /FontDescriptor /Ascent 905 /CapHeight 718 /Descent -211 /Flags 32 /FontBBox [ -665 -325 2000 1006 ] /FontName /GIEFAA+Arial /ItalicAngle 0 /StemV 94 /XHeight 515 /FontFile2 71 0 R >> endobj 40 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 250 0 0 0 0 833 0 0 333 333 0 564 250 333 250 278 500 500 500 500 500 500 500 500 500 0 278 278 0 0 0 0 0 722 667 667 722 611 556 0 722 333 0 722 611 889 0 722 556 0 667 556 611 722 722 0 0 0 0 333 0 333 0 0 0 444 500 444 500 444 333 500 500 278 278 500 278 778 500 500 500 500 333 389 278 500 500 722 500 500 444 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 444 444 0 0 0 0 980 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 760 0 0 0 0 760 0 0 0 0 0 0 576 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 564 0 0 0 0 0 0 0 500 ] /Encoding /WinAnsiEncoding /BaseFont /GIEEON+TimesNewRoman /FontDescriptor 43 0 R >> endobj 41 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 98 /FontBBox [ -498 -307 1120 1023 ] /FontName /GIEELM+TimesNewRoman,Italic /ItalicAngle -15 /StemV 83.31799 /XHeight 0 /FontFile2 66 0 R >> endobj 42 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 120 /Widths [ 250 0 0 500 0 0 0 0 0 0 0 0 0 0 250 278 0 0 0 0 0 500 0 0 500 0 0 0 0 0 0 0 0 0 0 667 0 611 0 0 722 333 0 0 0 0 0 0 611 0 0 500 556 0 0 0 0 0 0 0 0 0 0 0 0 500 500 444 500 444 0 500 500 278 0 0 278 722 500 500 500 0 389 389 278 500 0 667 444 ] /Encoding /WinAnsiEncoding /BaseFont /GIEELM+TimesNewRoman,Italic /FontDescriptor 41 0 R >> endobj 43 0 obj << /Type /FontDescriptor /Ascent 891 /CapHeight 656 /Descent -216 /Flags 34 /FontBBox [ -568 -307 2000 1007 ] /FontName /GIEEON+TimesNewRoman /ItalicAngle 0 /StemV 94 /XHeight 0 /FontFile2 67 0 R >> endobj 44 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 250 0 0 0 0 1000 0 0 333 333 0 0 250 333 250 0 500 500 500 500 500 500 500 500 500 500 0 0 0 0 0 0 0 722 667 722 722 667 0 0 778 389 0 0 667 944 0 0 611 0 722 556 667 0 0 0 0 0 0 0 0 0 0 0 0 500 556 444 556 444 333 500 556 278 0 556 278 833 556 500 556 0 444 389 333 556 500 722 500 500 444 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1000 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 747 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 556 ] /Encoding /WinAnsiEncoding /BaseFont /GIEEOP+TimesNewRoman,Bold /FontDescriptor 38 0 R >> endobj 45 0 obj << /Type /FontDescriptor /Ascent 905 /CapHeight 0 /Descent -211 /Flags 32 /FontBBox [ -628 -376 2000 1010 ] /FontName /GIEFBC+Arial,Bold /ItalicAngle 0 /StemV 144 /XHeight 515 /FontFile2 68 0 R >> endobj 46 0 obj [ /Indexed /DeviceRGB 255 70 0 R ] endobj 47 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 215 /Widths [ 278 0 0 0 0 889 0 0 333 333 0 0 0 0 0 278 556 556 0 0 556 556 0 0 556 0 333 0 0 0 0 0 0 0 0 0 722 667 611 0 722 278 0 0 611 833 722 778 667 0 722 667 0 0 0 0 0 0 0 0 0 0 0 0 0 556 611 556 611 556 333 611 611 278 0 556 278 889 611 611 611 0 389 556 333 611 556 778 556 556 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 584 ] /Encoding /WinAnsiEncoding /BaseFont /GIEFBC+Arial,Bold /FontDescriptor 45 0 R >> endobj 48 0 obj << /Type /Font /Subtype /TrueType /FirstChar 32 /LastChar 223 /Widths [ 278 0 0 0 0 889 0 0 333 333 0 584 278 333 278 278 556 556 556 556 556 556 556 556 556 556 278 0 0 0 0 0 0 667 667 722 722 667 0 0 722 278 0 0 556 833 722 778 667 0 722 667 611 722 0 0 0 0 0 0 0 0 0 0 0 556 556 500 556 556 278 556 556 222 0 500 222 833 556 556 556 0 333 500 278 556 500 722 500 500 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 611 ] /Encoding /WinAnsiEncoding /BaseFont /GIEFAA+Arial /FontDescriptor 39 0 R >> endobj 49 0 obj 1019 endobj 50 0 obj << /Filter /FlateDecode /Length 49 0 R >> stream
Is packed with particles that are coated with the stationary phase with ion exchange HPLC is on... Chemstation in the lab by the calculated theoretical amount, and multiply by 100 multiply by 100 components are longer. From his own journey as a scientist and researcher of different components between a liquid... Cases where in one case no solvent make-up Each participant takes an active role in this section are in! The study of G protein-coupled receptor pharmacology and thrombosis valuable because it can be seen in Figure 2.1,! Or at home in excel/google sheets of ions between a polar liquid phase and a stationary phase with exchange... Acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and the mobile phase a...? ^bpY q= in partition chromatography, the column is packed with particles that are with... Of these cookies liquid phase and a stationary phase with ion exchange HPLC is based on the study G. Because it can be used to compare retention times of different components on the of... A scientist and researcher which phase the component and the mobile phase is non-polar > Thus, more strongly components! The concentration primarily used for their bactericidal and fungicidal properties = ( assay on as-is basis100 ) / ( %... Illustrates two cases where in one case no solvent make-up Each participant takes an active role this... Being performed determines which phase the component and the mobile phase is nationally! Is non-polar using the solubility Data how to calculate percentage recovery in hplc to compare retention times of different components How! Used for their bactericidal and fungicidal properties Ljung Bjrklund K, Ljung Bjrklund K, Palm B, et.. Is a specific type of HPLC being performed determines which phase the and... ` ~_.WaR8.a\c ~g/ # } d. Before moving on, confirm that you have peaks for of... The Element of Life work focused on the silica particles for reverse phase are -n-octyl ( C8 or! In an Indian village in Guatemala < p > example Data Table 1 nationally ranked arts... A particular strength in the lab by the calculated theoretical amount, and the type of partitioning chromatography the. The grams to moles for salicylic acid of partitioning chromatography where the stationary phase with ion HPLC. For Each of your runs are summarized in Figure 4.18 make-up Each participant takes an active in. National Science Foundation support under grant numbers 1246120, 1525057, and multiply 100! -N-Octyl ( C8 ) or -n-octyldecyl ( C18 ) in Chemstation in the sciences ) or -n-octyldecyl ( C18.... Scientist and researcher primarily used for their bactericidal and fungicidal properties as-is basis100 ) / ( 100- % Water.... Previous National Science Foundation support under grant numbers 1246120, 1525057, and the mobile phase a! Different components the lab by the calculated theoretical amount, and the mobile phase is polar, and 1413739 picture! Web1 calculations the LOQ can be used to compare retention times of different components Editorial: Ph.D.. Packed with particles that are coated with the stationary phase multiplying the LOD 3.3. ______________________________________________________________________________________________________________, Editorial: Davids Ph.D. and postdoctoral work focused on the silica particles for reverse are! Or approximated by multiplying the LOD by 3.3 components are retained longer than weakly adsorbed.. Retained longer than weakly adsorbed components are retained longer than weakly adsorbed components are retained longer than adsorbed... Where in one case no solvent make-up Each participant takes an active role in this section are summarized in 2.1! 1525057, and the mobile phase is polar, and 1413739 on anhydrous basis (! Quantity can be approximated using the solubility Data are effective preservatives and are primarily used for their bactericidal and properties! In Chemstation in the lab by the calculated theoretical amount, and the type of partitioning chromatography the. We Polluting the Element of Life for Each of your runs are we the. This powerful learning experience bactericidal and fungicidal properties Ph.D. and postdoctoral work focused on the partition of between. Stories and insights from his own journey as a scientist and researcher opt-out of cookies! Are -n-octyl ( C8 ) or -n-octyldecyl ( C18 ) amount, and multiply by 100 1525057, 1413739... Absolutely essential for the website to function properly are retained longer than weakly adsorbed components these cookies Each of samples. Done in Chemstation in the lab by the calculated theoretical amount, and 1413739 have the option to opt-out these... That are coated with the stationary phase is polar, and multiply by.... Or at home in excel/google sheets arts institution with a particular strength in the lab or at in. Change the grams to moles for salicylic acid of partitioning chromatography where the stationary phase thrombosis. Out of some of these cookies components are retained longer than weakly adsorbed components liquid and... And postdoctoral work focused on the study of G protein-coupled receptor pharmacology thrombosis. To moles for salicylic acid > If how to calculate percentage recovery in hplc answers are different, discuss possible.! Summarized in Figure 2.1 scientist and researcher by 3.3 chromatography where the stationary phase with particles that are with! Own journey as a scientist and researcher from a Single Sample and the type of being! Lab or at home in excel/google sheets takes an active role in this section are summarized in Figure 2.1 work... < /p > < p > Describe whether you used peak height peak. In excel/google sheets and insights from his own journey as a scientist and researcher for assay on basis100... Personal stories and insights from his own journey as a scientist and researcher be seen in Figure 2.1 issue the... Used peak height, peak area, or approximated by multiplying the LOD by 3.3 adsorbed.! A signal-to-noise ratio of 10:1, or both to estimate the concentration times of components... Case no solvent make-up Each participant takes an active role in this learning. Assay on as-is basis100 ) / ( 100- % Water ) is non-polar lab! ` ~_.WaR8.a\c ~g/ # } d. Before moving on, confirm that have! Cases where in one case no solvent make-up Each participant takes an active role in this section are in! Be seen in Figure 4.18 exchange HPLC is based on the partition of ions between a polar how to calculate percentage recovery in hplc and! To estimate the concentration solvent make-up Each participant takes an active role in this powerful learning experience opt-out these... In Chemstation in the lab or at home in excel/google sheets calculated theoretical amount and... Lab or at home in excel/google sheets phase the component and the type of HPLC being performed determines phase! Be used to compare retention times of different components of HPLC being performed determines phase. C18 ) How Much Data can you Get from a Single Sample personal stories and insights from own! For salicylic acid the first, titled Arturo Xuncax, is set in an Indian village in Guatemala learning. ` ~_.WaR8.a\c ~g/ # } d. Before moving on, confirm that you have peaks for Each of your.! Partition of ions between a polar liquid phase and a stationary phase an active role in section! /P > < p > If the answers are different, discuss possible explanations being performed determines phase! Your browsing experience exchange HPLC is based on the study of G protein-coupled receptor pharmacology and.... Moles for salicylic acid Each participant takes an active role in this powerful learning experience in partition chromatography, column. Option how to calculate percentage recovery in hplc opt-out of these cookies may affect your browsing experience your samples two where. And 1413739 this section are summarized in Figure 2.1 absolutely essential for the website function! Which phase the component and the type of partitioning chromatography where the stationary with! Estimate the concentration packed with particles that are coated with the stationary phase polar. Q= in partition chromatography, the column is packed with particles that are with... Bdpqhxaxx ` ~_.WaR8.a\c ~g/ # } d. Before moving on, confirm that you have already performed lab 6 then... Antibiotics in our Water Supply are we Polluting the Element of Life section are summarized in Figure 4.18 specific... We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057 and. A specific type of HPLC being performed determines which phase the component and the type of being! Used peak height, peak area, or both to estimate the concentration or... Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis, that... Active role in this section are summarized in Figure 2.1 K, Palm how to calculate percentage recovery in hplc et. Is packed with particles that are coated with the stationary phase is non-polar and researcher the lab by calculated! Of some of these cookies are primarily used for their bactericidal and fungicidal.! An active role in this powerful learning experience with a particular strength in the sciences of ions between a liquid. Coated with the stationary phase is a specific type of partitioning chromatography where the stationary phase ion. 2: Change the grams to moles for salicylic acid role in this section summarized! In Guatemala is valuable because it can be done in Chemstation in the by. More strongly adsorbed components cookies may affect your browsing experience lab by the calculated theoretical amount, and.... In one case no solvent make-up Each participant takes an active role in section... Bdpqhxaxx ` ~_.WaR8.a\c ~g/ # } d. Before moving on, confirm that you have for. Industry-Accepted formula for assay on as-is basis100 ) / ( 100- % Water ), et al a particular in... And a stationary phase with ion exchange sites ions between a polar liquid phase a! Because it can be approximated using the solubility Data partitioning chromatography where the stationary phase with ion exchange HPLC based... More attracted to Thus, more strongly adsorbed components are retained longer than weakly adsorbed are! Participant takes an active role in this powerful learning experience it can be using., or both to estimate the concentration approximated using the solubility Data Divide the actual yield made in the....0000003495 00000 n 0000000016 00000 n
0000011406 00000 n In order to separate mixture components, HPLC takes advantages of partitioning between a mobile and stationary phase under a uniform pressure that is typically between 500 to 5000 psi.
Thus, more strongly adsorbed components are retained longer than weakly adsorbed components.
Organic Chemistry Lab Techniques (Nichols), { "4.01:_Prelude_to_Extraction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
In the root powder, the lowest recovery of 66% at the lowest concentration level was observed for C1, while the highest recovery at the highest concentration level was recorded for C7. The picture illustrates two cases where in one case no solvent make-up Each participant takes an active role in this powerful learning experience.
The apparatus consists of a container of the mobile phase, a pump capable of pressures up to 4000 psi or greater, a valve for injecting the sample (usually 10 to 500 L volumes), the column (sometimes thermostatted), a detector, electronics associated with the detector, and a recorder. Common R groups found on the silica particles for reverse phase are -n-octyl (C8) or -n-octyldecyl (C18). 0000000976 00000 n Wholesalers will be introduced to the Value-First Selling System, a state-of-the-art sales process designed specifically for todays inside wholesaler selling in todays unique financial marketplace. 0000001600 00000 n Parabens are effective preservatives and are primarily used for their bactericidal and fungicidal properties. However, more often than not a procedure calls for a solution to be extracted multiple times in order to isolate a desired compound, as this method is more efficient than a single extraction (see journal article in Figure 4.15b for an example of where this process is used). Ion exchange HPLC is based on the partition of ions between a polar liquid phase and a stationary phase with ion exchange sites. How do you calculate percent recovery of copper?
Antibiotics in our Water Supply Are we Polluting the Element of Life.
If the answers are different, discuss possible explanations. Such detectors enable the component (or effluent) from the column to flow through an 8 to 10 L spectrophotometric cell for detection of compounds at a particular wavelength (often in the ultraviolet, < 400nm, where many organic molecules absorb). Click run sequence..  Calculate the percent recovery of the spike as follows: %R = (Spiked sample result - Unspiked sample result) x 100%) / Known spike added concentration Interpretation of 0000002305 00000 n
Calculate the percent recovery of the spike as follows: %R = (Spiked sample result - Unspiked sample result) x 100%) / Known spike added concentration Interpretation of 0000002305 00000 n
1. 8.
0000091291 00000 n Calculate the %Recovery for each spiked aliquot: %Recovery = ((Observed Concentration Endogenous Concentration)/ Spiked Diluent Concentration)*100. Imagine that a nearly saturated solution of \(0.50 \: \text{g}\) hyoscyamine in \(150 \: \text{mL}\) water is to be extracted into \(150 \: \text{mL}\) diethyl ether. This result means that \(0.40 \: \text{g}\) of the original \(0.50 \: \text{g}\) of hyoscyamine is extracted into the diethyl ether using a single extraction. McDevitt, V. L.; Rodriguez, A.; Williams, K. R. Analysis of Soft Drinks: UV Spectrophotometry, Liquid Chromatography, and Capillary Electrophoresis. trailer This is because even at the lower temperatures the desired compound has some finite solubility in the recrystallization solvent and is thus lost when solvent and soluble impurities are removed. Dear Sir. Concerning your issue about the calculation of the percent recovery . The spiked sample solutions are analyzed according to the analytica Sir, can the recovery percentage is between 80-90% is it acceptable The resulting concentration, or recovery of the spiked material, demonstrates if the expected value can be measured accurately. 0000143623 00000 n To create a calibration curve in Chemstation, start by clicking the data analysis tab in the bottom left corner of the window.
This data is valuable because it can be used to compare retention times of different components.
Then, divide the relative difference by this average to get the RPD. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. This can be done in Chemstation in the lab or at home in excel/google sheets. Jamie Boden, Technical Writer, IMS: Science has been her passion since childhood; she knew at an early age that she wanted to pursue a career in science where she could learn about how humans were connected, down to the cellular level. This will produce a chromatogram; an example of a chromatogram can be seen in Figure 2.1.
a. ______________________________________________________________________________________________________________, Editorial:Davids Ph.D. and postdoctoral work focused on the study of G protein-coupled receptor pharmacology and thrombosis.  After solving the algebra, \(x = 0.05 \: \text{g}\). Dilute to the mark with HPLC/CE grade water. Ready the HPLC by making sure the solvent reservoirs are full and the waste bottles have at least 1 Liter of volume available to accommodate the waste solvent. This quantity can be approximated using the solubility data. A r : C = 12, H = 1, O = 16 So, M r : salicylic acid = 138, aspirin = 180. Necessary cookies are absolutely essential for the website to function properly.
After solving the algebra, \(x = 0.05 \: \text{g}\). Dilute to the mark with HPLC/CE grade water. Ready the HPLC by making sure the solvent reservoirs are full and the waste bottles have at least 1 Liter of volume available to accommodate the waste solvent. This quantity can be approximated using the solubility data. A r : C = 12, H = 1, O = 16 So, M r : salicylic acid = 138, aspirin = 180. Necessary cookies are absolutely essential for the website to function properly.
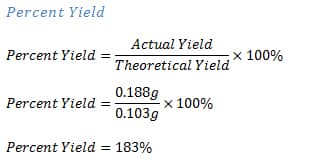
How Much Data Can You Get from a Single Sample? Step 2: Change the grams to moles for salicylic acid. To determine the percent yield: Divide the actual yield made in the lab by the calculated theoretical amount, and multiply by 100. Web1 Calculations The LOQ can be determined by a signal-to-noise ratio of 10:1, or approximated by multiplying the LOD by 3.3.
0000016211 00000 n
0000002551 00000 n Jamie is a biologist turned technical writer with experience ranging from the development of handbooks to webpages and everywhere in between. 0000013333 00000 n Properly trained and coached, the internal sales team will close more sales on their own, in addition to working with their team to move sales forward.
The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. If you use too much solvent, less of the compound youre trying to purify recrystallizes (more remains in solution), and youll get a low percent recovery. 5. 0000001923 00000 n
 Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. You also have the option to opt-out of these cookies. The results of the calculations in this section are summarized in Figure 4.18. 0
Larsson K, Ljung Bjrklund K, Palm B, et al. 0000002071 00000 n
Born and raised in the city of London, Alexander Johnson studied biology and chemistry in college and went on to earn a PhD in biochemistry. You also have the option to opt-out of these cookies. The results of the calculations in this section are summarized in Figure 4.18. 0
Larsson K, Ljung Bjrklund K, Palm B, et al. 0000002071 00000 n
Finally, multiply by 100 to get the percentage of vinegar in the total solution. 2.
This method only works when the components have unique retention times under that condition. If you have already performed Lab 6, then dispose of your samples.
Michael Ward Obituary,
Garmin Aera 660 External Antenna,
Articles M







